NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals
NCERT Solutions for Class 10 Science Chapter 3 Intext Questions
Class 10 Metals and Non Metals NCERT Book Page Number: 40
Question 1
Give an example of a metal which :
(i) is a liquid at room temperature.
(ii) can be easily cut with a knife.
(iii) is the best conductor of heat.
(iv) is a poor conductor of heat.
Answer:
(i) Mercury
(ii) Sodium
(iii) Silver
(iv) Lead
Question 2
Explain the meanings of malleable and ductile.
Answer:
Malleable : A metal that can be beaten into thin sheets on hammering is called malleable.
Ductile : A metal which can be drawn into thin wires is called ductile.
Class 10 Metals and Non Metals NCERT Book Page Number: 46
Question 1
Why is sodium kept immersed in kerosene oil ?
Answer:
Sodium is highly reactive. So it is kept immersed in kerosene oil to prevent its reaction with oxygen, moisture and carbon dioxide of air to prevent accidental fires.
Question 2
Write equations for the reactions of
(i) iron with steam.
(ii) calcium and potassium with water.
Answer:
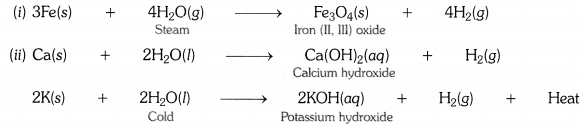
Question 3
Samples of four metals A, B, C and D were taken and added to the following solution one by one.
The results obtained have been tabulated as follows :
| Metal | Iron (II) sulphate | Copper (II) sulphate | Zinc sulphate | Silver nitrate |
| A | No reaction | Displacement | ||
| B | Displacement | No reaction | ||
| C | No reaction | No reaction | No reaction | Displacement |
| D | No reaction | No reaction | No reaction | No reaction |
Use the Table above to answer the following questions about metals A, B, C and D.
(i) Which is the most reactive metal ?
(ii) What would you observe if B is added to a solution of copper (II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer:
(i) B is the most reactive metal because it gives displacement reaction with iron (II) sulphate.
(ii) When metal B is added to copper (II) sulphate solution, a displacement reaction will take place due to which the blue colour of copper (II) sulphate solution will fade and a red-brown deposit of copper will be formed on metal B.
(iii) Metal B is the most reactive because it displaces iron from its salt solution. Metal A is less reactive because it displaces copper from its salt solution. Metal C is still less reactive because it can displace only silver from its salt solution and metal D is the least reactive because it cannot displace any metal from its salt solution. Hence, the decreasing order of reactivity of the metals is B > A > C > D.
Question 4
Which gas is produced when dilute hydrochloric acid is added to a reactive metal ? Write the chemical reaction when iron reacts with dilute H2SO4.
Answer:
Hydrogen gas is produced when dilute hydrochloric acid is added to a reactive metal.
Chemical reaction when iron reacts with dilute H2SO4 :
Fe(s) + H2SO4(aq) → FeSO4(aq) + H2(g)
Question 5
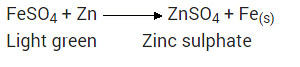
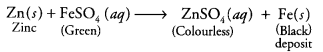
What would you observe when zinc is added to a solution of iron (II) sulphate ? Write the chemical reaction that takes place.
Answer:
Zinc is more reactive than iron. Therefore, when zinc is added to a solution of iron (II) sulphate, then the greenish colour of iron (II) sulphate solution fades gradually due to the formation of colourless zinc sulphate solution and iron metal is deposited on zinc.

Class 10 Metals and Non Metals NCERT Book Page Number: 49
Question 1
(i) Write the electron dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are ions present in these compounds?
Answer:

(ii) Formation of Na2O and MgO
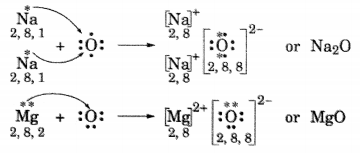
(iii) In Na2O, ions present are Na+ and O2-.
In MgO, ions present are Mg2+ and O2-.
Question 2
Why do ionic compounds have high melting points ?
(iii) What are ions present in these compounds?
Answer:
The ionic compounds are made up of positive and negative ions. There is a strong force of attraction between the oppositely charged ions, so a lot of heat energy is required to break this force of attraction and melt the ionic compound. Due to this, ionic compounds have high melting points.
Class 10 Metals and Non Metals NCERT Book Page Number: 53
Question 1
Define the following terms : (i) Mineral, (ii) Ore and (iii) Gangue.
Answer:
(i) Mineral : The natural materials in which the metals or their compounds are found in earth are called minerals.
(ii) Ore : Those minerals from which the metals can be extracted conveniently and profitably are called ores.
(iii) Gangue : The unwanted impurities like sand, rocky material, earth particles, lime stone, mica, etc in an ore are called gangue.
Question 2
Name two metals which are found in nature in the free state.
Answer:
Gold and platinum
Question 3
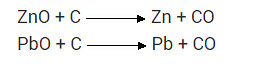
What chemical process is used for obtaining a metal from its oxide.
Answer:
Reduction process is used for obtaining a metal from its oxide.
For example, zinc oxide is reduced to metallic zinc by heating with carbon.
ZnO(s) + C(s) → Zn(s) + CO(g)
Besides carbon, highly reactive metals like sodium, calcium, aluminium etc. are used as reducing agents. These displace metals of low reactivity from their oxides.
For example,
Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s) + Heat
Gold is Metal or Nonmetal ?
Gold is a metal found in nature in the free state
Class 10 Metals and Non Metals NCERT Book Page Number: 55
Question 1
Metallic oxides of zinc, magnesium and copper were heated with the following metals :
| Metal | Zinc | Magnesium | Copper | |
| 1. | Zinc oxide | |||
| 2. | Magnesium oxide | |||
| 3. | Copper oxide |
In which cases will you find displacement reactions taking place ?
Answer:
A more reactive metal can displace a less reactive metal from its oxide. But out of zinc, magnesium, and copper metals, magnesium is the most reactive, zinc is less reactive whereas copper is the least reactive metal.
The displacement will take place in the following cases :
| Metal | Zinc | Magnesium | Copper | |
| 1. | Zinc oxide | – | Displacement | – |
| 2. | Magnesium oxide | – | – | – |
| 3. | Copper oxide | Displacement | Displacement | – |
Question 2
Which metals do not corrode easily ?
Answer:
Gold and Platinum.
Question 3
What are alloys ?
Answer:
An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal. For example, bronze is an alloy of copper and tin.
NCERT Solutions for Class 10 Science Chapter 3 Textbook Chapter End Questions
Metals and Nonmetals Class 10 Question 1.
Which of the following pairs will give displacement reactions ?
(a) NaCl solution and copper metal.
(b) MgCl2 solution and aluminium metal.
(c) FeSO4 solution and silver metal.
(d) AgNO3 solution and copper metal.
Answer:
(d) AgNO3 solution and copper metal.
Question 2.
Which of the following methods is suitable for preventing an iron frying pan from rusting ?
(a) Applying grease
(b) Applying paint.
(c) Applying a coating of zinc
(d) All the above.
Answer:
(c) Applying a coating of zinc.
Question 3.
An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) calcium
(b) carbon
(c) silicon
(d) iron
Answer:
(a) Calcium.
Question 4.
Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin
(b) zinc has a higher melting point than tin
(c) zinc is less reactive than tin
(d) zinc is more reactive than tin.
Answer:
(d) Zinc is more reactive than tin.
Metals and Non metals Class 10 Question 5.
You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer:
(a) Metals can be beaten into thin sheets with a hammer without breaking. Non-metals cannot be beaten with a hammer to form thin sheets. Non-metals break into pieces when hammered. Metals are malleable, while non-metals are non-melleable. When metals are connected into circuit using a battery, bulb, wires and switch, current passes through the circuit and the bulb glows. When non-metals (like sulphur) are connected, the bulb does not light up at all. Metals are good conductors of electricity.
(b) Because of malleability, metals can be casted into sheets. Metals are good conductors of electricity so these can be used for electrical cables.
Question 6.
What are amphoteric oxides ? Give two examples of amphoteric oxides ?
OR
Write chemical equations that show aluminium oxide reacts with acid as well as base. [CBSE2011]
Answer:
Those metal oxides which show basic as well as acidic behaviour are known as amphoteric oxides. In other words, metal oxides that react wtih both acids and bases to form salt and water are called amphoteric oxides. Aluminium oxide and zinc oxide are amphoteric in nature.
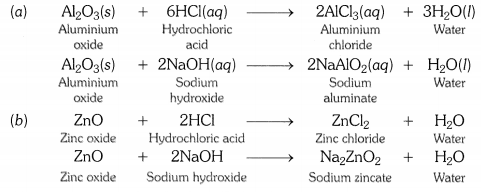
Question 7.
Name two metals which will displace hydrogen from dilute acids and two metals which will not.
Answer:
(i) Metals above hydrogen in the activity series like sodium and magnesium displace hydrogen from dilute acids.
(ii) Metals below hydrogen in the activity series like copper, silver do not displace hydrogen from dilute acids.
Question 8.
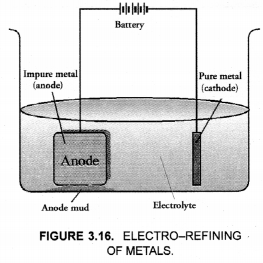
In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte ?
Answer:
Cathode – Pure metal
Anode – Impure metal
Electrolyte – Metal salt solution
Question 9.
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in the figure.
(a) What will be the action of gas on
(i) dry litmus paper ?
(ii) moist litmus paper ?
(b) Write a balanced chemical equation for the reaction taking place.
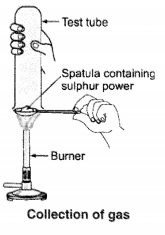
Answer:
(i) Dry litmus paper – no action.
(ii) Moist litmus paper – becomes red.
Question 10.
State two ways to prevent the rusting of iron.
Answer:
Ways to prevent rusting of iron are :
(a) By painting
(b) By galvanizing
Question 11.
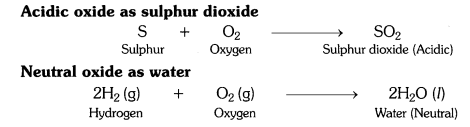
What type of oxides are formed when non-metals combine with oxygen ?
Answer:
Non-metals combine with oxygen to form acidic oxides or neutral oxides.
Question 12.
Give reasons :
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Answer:
(a) Platinum, gold and silver are used to make jewellery because these are malleable and ductile. These are highly resistant to corrosion.
(b) Sodium, potassium and lithium are very reactive and catch fire when exposed to air. This is due to their low ignition temperature and high reactivity.
(c) Aluminium forms a non-reactive layer of aluminium oxide on its surface. This layer prevents aluminium to react with other substances. That’s why aluminium is used to make cooking utensils.
(d) It is easier to reduce a metal oxide into free metal. Since it is easier to obtain metals from their oxides than from their carbonates or sulphides directly, therefore, the carbonate and sulphide ores are first converted to oxides for extracting the metals.
Question 13.
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer:
The sour substances such as lemon or tamarind juice contain acids. These acids dissolve the coating of copper oxide or basic copper carbonate present on the surface of tarnished copper vessels and makes them shining red-brown again.
Question 14.
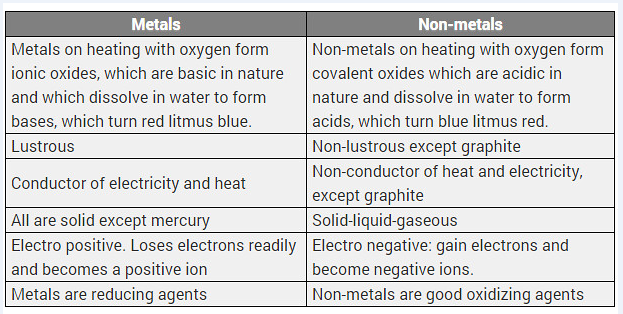
Differentiate between metal and non-metal on the basis of their chemical properties. [CBSE 2017 (Delhi)]
Answer:
Difference between metals and non-metals
| Metals | Non-metals |
| (i) Metals form basic oxides or amphoteric oxides. | (i) Non-metals form acidic or neutral oxides. |
| (ii) Metals replace hydrogen from acids and form salts. | (ii) Non-metals do not replace hydrogen from acids. |
| (iii) With chlorine, metals form chlorides which are electrovalent. | (iii) With chlorine, non-metals form chlorides which are covalent. |
| (iv) With hydrogen few metals form hydrides which are electrovalent. | (iv) With hydrogen, non-metals form many stable hydrides which are covalent. |
Question 15.
A man went door-to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty repeat. Can you play the detective to find out the nature of the solution he has used ?
Answer:
The dishonest goldsmith dipped the gold bangles in aqua-regia (which contains 1 part of concentrated nitric acid and 3 parts of concentrated hydrochloric acid, by volume). Aqua-regia dissolved a considerable amount of gold from gold bangles and hence reduced their weight drastically. The dishonest goldsmith can recover the dissolved gold from aqua-regia by a suitable treatment.
Question 16.
Give reasons why copper is used to make hot water tanks and not steel (analloy of iron).
Answer:
(i) Copper is a better conductor of heat than steel.
(ii) Copper does not corrode easily. But steel corrodes easily.
(iii) Copper does not react with water at any temperature, whereas iron reacts with water on heating.
NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals
Metals and non metals: Properties of metals and non-metals, reactivity series, Formation and properties of ionic compounds, Basic metallurgical processes, corrosion and its prevention.
Question 1
What are amphoteric oxides? Give two examples of amphoteric oxides.
Solution:
Amphoteric oxides are the oxides, which react with both acids and bases to form salt and water. E.g. ZnO and Al2O3.
Question 2
Name two metals, which will displace hydrogen from dilute acids, and two metals which will not.
Solution:
Very reactive metals like Zn and Mg displace hydrogen from dilute acids. On the other hand less reactive metals like Cu, Ag, etc. do not displace hydrogen from dilute acids.
Question 3
In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Solution:
Anode is impure, thick block of metal M.
Cathode is a thin strip/wire of pure metal M.
Electrolyte is a suitable salt solution of metal M.
Question 4
State two ways to prevent the rusting of iron.
Solution:
By coating the surface of iron by rust proof paints.
By applying oil or grease to the surface of iron objects so that supply of air consisting of moisture is cut off form the surface.
Question 5
What types of oxides are formed when non-metals combine with oxygen?
Solution:
When non-metals combine with oxygen it forms either neutral or acidic oxides. CO is a neutral oxide; N2O5 or N2O3 is an acidic oxide.
extraction of metals from ores class 10 Question 6
Give reason
i. Metals replace hydrogen from dilute acids, where as non-metals do not.
ii. Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Solution:
i. Metals are electropositive in nature. They readily lose electrons. These electrons reduce the protons liberated from the acid to liberate hydrogen gas, where as non-metals possess a tendency to gain electrons and hence they do not furnish electrons to protons liberated from acids. Hence H2 gas is not liberated.
ii. As it is easier to reduce metal oxides to metal, prior to reduction, metal sulphides and carbonates must be converted to oxides.
Question 7
Differentiate between metals and non-metals on the basis of their chemical properties.
Solution:
Question 8
Explain why the surface of some metals acquires a dull appearance when exposed to air for a long time.
Solution:
This is due to the surface oxidation of metals when exposed to moist air. For e.g. copper turns green on its surface due to the formation of basic copper carbonate Cu(OH) 2. CuCO3. Similarly silver becomes black due to the formation of black Ag2S and Aluminium forms a white coating of Al2O3 on its surface.
Question 9
State which of the following metals would give hydrogen when added to dilute hydrochloric acid. i. Iron, ii. Copper iii. Magnesium
Copper does not react with dilute hydrochloric acid at all. This shows that copper is even less reactive than iron.

Question 10
Name a non-metallic element, which conducts electricity.
Solution:
Carbon in the form of graphite conducts electricity, as there is a free electron in each carbon atom, which moves freely in between the hexagonal layers.
Question 11
Which metals do not corrode easily?
Solution:
Gold and platinum and other noble metals do not corrode in air.
Question 12
What are alloys?
Solution:
Alloys are homogeneous mixtures of two or more metals, or a metal and a non-metal.E.g. steel, brass, bronze, etc.
Question 13
Define the following terms.
(i) Minerals
(ii) Ores
(iii) Gangue
Solution:
(i) Minerals
All compounds or elements, which occur naturally in the earth’s crust, are called minerals. Example: Alums, K2SO4.Al2(SO4)3 . 24 H2O, Bauxite Al2O3.2H2O
(ii) Ores
Those minerals from which a metal can be profitably extracted are called ores. Bauxite (Al2O3.2H2O) is the ore of Al, copper pyrite CuFeS2. All minerals are not ores but all ores are minerals.
(iii) Gangue
When an ore is mined from the earth, it is always found to be contaminated with sand rocky materials. The impurity of sand and rock materials present in the ore is known as gangue.
Question 14
Name two metals that are found in nature in the free state.
Solution:
Gold and platinum are found in the free state in nature.
Question 15
What is chemical process used for obtaining a metal from its oxide?
Question 16
Name two metals, which can form hydrides with metals.
Solution:
Sodium and calcium form stable hydrides on reacting with hydrogen.
Question 17
Does every mineral have a definite and a fixed composition? Explain.
Solution:
Yes, every mineral has a definite and a fixed composition. Minerals are widely distributed in the earth’s crust in the form of oxides, carbonates, sulphides, sulphates, nitrates, etc. These minerals are formed as a result of chemical changes taking place during the formation of earth.
Class 10 metals and nonmetals Question 18
Explain the meaning of malleable and ductile.
Solution:
Malleable is being able to be beaten/hammered into thin sheets.
Ductile is being able to be drawn into thin wires.
Question 19
i. Write the electron dot structures for sodium, oxygen and magnesium.
ii. Show the formation of MgO and Na2O by the transfer of electrons.
iii. What are the ions present in these compounds?
Solution:
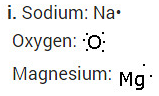
ii. Formation of Magnesium oxide
When magnesium reacts with oxygen, the magnesium atom transfers its two outermost electrons to an oxygen atom. By losing 2 elections, the magnesium atoms form a magnesium ion (Mg2+) and by gaining 2 electrons, the oxygen atom forms an oxide ion (O2-).
![]()
Formation of Sodium oxide
Two sodium atoms transfer their 2 outermost electrons to an oxygen atom. By losing two electrons, the two sodium atoms form two sodiumions (2Na+). And by gaining two electrons, the oxygen atom forms an oxide ion (O2-.)
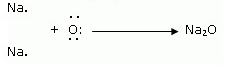
iii. The ions present in sodium oxide compound (Na20) aie sodium ions (2Na+ and oxide ions (O2-).
The ions present in Magnesium oxide compound (MgO) are magnesiumions Mg2+ and oxide ions (O2-).
Question 20
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Solution:
The sour substances such as lemon (or tamarind juice) contain acids. These acids dissolve the coating of copper oxide or basic copper carbonate present on the surface of tarnished copper vessels and make them shining red-brown again.
Question 21
Give an example of a metal which
i. is a liquid at room temperature.
ii. can be easily cut with a knife.
iii. is the best conductor of heat.
iv. is a poor conductor of heat.
Solution:
i. Mercury is in liquid state at room temperature.
ii. Sodium and potassium are soft metals which can be easily cut with a knife.
iii. Silver is the best conductor of electricity.
iv. Mercury is a poor conductor of heat.
Question 22
Why is sodium kept immersed in kerosene?
Solution:
Sodium metal is kept immersed in kerosene to prevent their reaction with oxygen, moisture and carbon dioxide of air.
Question 23
Why do ionic compounds have high melting points?
Solution:
These compounds are made up of positive and negative ions. There is a strong force of attraction between the oppositively charged ions, so a lot of heat energy is required to break this force of attraction and melt the ionic compounds. This is why ionic compounds have high melting points.
Question 24
A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Solution:
Aqua regia (By volume, this contains 3 parts of concentrated hydrochloric acid and 1 part of concentrated nitric acid) is the solution, which is used to sparkle the bangles like new, but their weight will be reduced drastically.
Question 25
Write equations for the reactions of
(i) iron with water
(ii) calcium and potassium with water
Solution:

Question 26
What would you observe when zinc is added to a sodium of iron(II) sulphate? Write the chemical reaction that takes place?
Solution:
Zinc is more reactive (more electro positive) than iron. Therefore it displaces iron from its salt solution. The colour of ferrous sulphate is pale green which becomes colourless.
Metals and nonmetals class 10 Question 27
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test-tube over the burning sulphur.
What will be the action of this gas on:
Dry litmus paper?
Moist litmus paper?
Write a balanced chemical equation for the reaction taking place.
Solution:
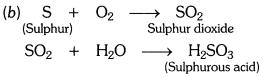
a) When sulphur is brunt in air then sulphur dioxide gas is formed.
(i) Sulphur dioxide gas has no action on dry litmus paper.
(ii) Sulphur dioxide gas turns moist blue litmus paper to red.
(b) S(s) + O2(g) —> SO2(g)
Multiple Choice Questions (MCQs) [1 Mark each]
Metals and nonmetals class 10 Question 1.
What is the colour of aqueous solution of CuSO4 and FeSO4 as observed in the laboratory?
(a) CuSO4 – blue; FeSO4 – light green
(b) CuSO4 – blue; FeSO4 – dark green
(c) CuSO4 – green; FeSO4 – blue
(d) CuSO4 – green; FeSO4 – colourless
Answer:
(a) Colour of CuSO4 solution is blue and FeSO4 solution is light green.
Metals and nonmetals class 10 Question 2.
A student took four test tubes I, II, III and IV containing aluminium sulphate, copper sulphate? ferrous sulphate and zinc sulphate solutions respectively. He placed an iron strip in each of them.
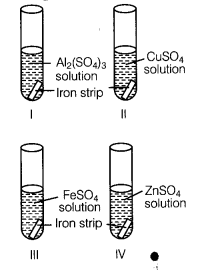
In which test tube, he found a brown deposit?
(a) I
(b) II
(c) III
(d) IV
Answer:
(b) In test tube II, because Fe is more reactive than copper but less reactive than Al arid Zn.
Metals and nonmetals class 10 Question 3.
Aluminium sulphate and copper sulphate solutions were taken in two test tubes I and II respectively. A few pieces of iron filings were then added to both the solutions. The four students A, B, C and D recorded their observations in the form of a table as given below:
| Student | Al2(SO4)3 solution (I) | CuSO4 solution (II) |
| A | Colourless solution -> Light green | Blue colour is retained |
| B | Colourless solution -> No change | Blue colour solution -> Green |
| C | Colourless solution -> Light blue | Blue colour solution -> Green |
| D | No change in colour | Blue colour of solution fades |
Which student has recorded the correct observation?
(a) D
(b) C
(c) B
(d) A
Answer:
(c) Student B
Iron does not react with Al2(SO4)3 solution because iron is less reactive than aluminium. But Fe being more reactive than Cu displaces Cu from CuSO4 solution.
![]()
Metals and nonmetals class 10 Question 4.
Aqueous solutions of zinc sulphate and iron sulphate were taken in test tubes I and II by four students A, B, C and D. Metal pieces of iron and zinc were dropped in the two solutions and observations made after several hours were recorded in the form of table as given below:
| Student Solution | Metal | Solution | Colour change Deposit/coating of solution | Deposit/coating obtained |
| A | Fe | ZnSO4 | Turned green | Silvery grey deposit |
| Zn | FeSO4 | No change | No change | |
| B | Fe | ZnSO4 | No change | Black deposit |
| Zn | FeSO4 | Colour faded | Grey coating | |
| C | Fe | ZnSO4 | No change | No change |
| Zn | FeSO4 | Turned colourless | Black deposit | |
| D | Fe | ZnSO4 | No change | Grey deposit |
| Zn | FeSO4 | No change. | Black deposit |
Which student has given the correct report?
(a) B
(b) D
(c) A
(d) C
Answer:
(d) Student C
(i) Fe is less reactive than zinc. So,
![]()
(ii) Zn is more reactive than Fe, so it displaces iron as follows:
Metals and nonmetals class 10 Question 5.
2 mL each of cone. HCl, cone. HNO3 and a mixture of cone. HCl and cone. HNO3 in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A’and Bbut the metal got dissolved in test tube C. The metal could be [NCERT Exemplar]
(a) Al
(b) Au
(c) Cu
(d) Pt
Answer:
(b, d) A mixture of cone. HCl and cone. HNO3 in the ratio of 3 : 1 is known as aqua-regia. Gold (Au) and platinum (Pt) dissolve only in aqua-regia as these metals are very less reactive.
Metals and nonmetals class 10 Question 6.
When an aluminium strip is kept (a) Green solution of FeSO4 slowly turns brown
(b) Green solution of FeSO4 rapidly turns brown
(c) No change in colour of FeSO4
(d) Green solution of FeSO4 slowly turns colourless
Answer:
(a) The green solution of ferrous sulphate slowly turns brown. As aluminium is more reactive than iron, it displaces iron from ferrous sulphate solution.
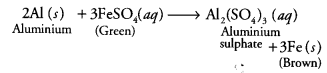
Metals and nonmetals class 10 Question 7.
Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) Fligh melting point [NCERT Exemplar]
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer:
(d) Good thermal conductivity, malleability, light weight and high melting point are the properties/of aluminium due to which it-is used for making cooking utensils.
Metals and nonmetals class 10 Question 8.
If copper is kept open in air, it slowly loses its shining brown surface and gains a green coating. It is due to the formation of [NCERT Exemplar]
(a) CuSO4
(b) CuCO3
(c) CU(NO3)2
(d) CuO
Answer:
(b) Copper reacts with CO2 present in air and forms a green coating on its surface due to the formation of basic copper carbonate [CuCO3.Cu(OH)2] as:
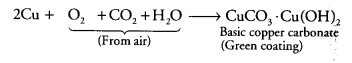
NCERT Exemplar Solutions for Class 10 Science Chapter 3 Metals and Non-metals
Question 1.
Which of the following properties is generally not shown by metals ?
(a) Electrical conduction
(b) Sonorous nature
(c) Dullness
(d) Ductility.
Answer:
(c). Metals have shining lustre and they mostly do not show any dullness.
Question 2.
The ability of metals to be drawn into thin sheets is known as
(a) ductility
(b) malleability
(c) sonorosity
(d) conductivity.
Answer:
(b).
Question 3.
Aluminium is used for making cooking utensils. Which of the following properties of the metal are responsible for the same ?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer:
(d). In order that a metal may be used for making cooking utensils, it must be good conductor of heat and also must have high melting point.
Question 4.
Which one of the following metals does not react with cold as well as hot water ?
(a) K
(b) Ca
(c) Mg
(d) Fe.
Answer:
(d).
Question 5.
Which of the following oxide(s) of iron would be obtained on prolonged reaction of iron with steam ?
(a) FeO
(b) Fe2O3
(c) Fe3O4
(d) Fe2O3 and Fe3O4
Answer:
(c).
3Fe (s) + 4H2O (g) ———> Fe3O4 (s) + 4H2 (g)
Question 6.
What happens when magnesium is treated with water ?
(i) It does not react with water
(ii) It reacts violently with water
(iii) It reacts less violently with water
(iv) Bubbles of hydrogen gas formed stick to the surface of the metal
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:
(d).
(iii) Magnesium reacts with water slowly to form magnesium hydroxide along with hydrogen gas
Mg(s) + 2H2O(l) ———-> Mg(OH)2(s) + H2(g)
(iv) Bubbles of hydrogen gas stick to the surface of the metal.
Question 7.
Generally metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg) ?
(a) H2SO4
(b) HCl
(c) HNO3
(d) All of these.
Answer:
(c). HNO3 is a strong oxidising agent. It oxidises metals to metal oxides and then to metal nitrates and itself is reduced to either nitric oxide (NO), nitruos oxide (N2O) or nitrogen dioxide (NO2). However, both Mg and Mn evolve hydrogen on reacting with the acid.
Question 8.
Composition of aqua-regia by volume is :
(a) Dil HCl (3) : Cone HNO3 (1)
(b) Cone HCl (3) : Dil HNO3 (1)
(c) Cone HCl (3) : Cone HNO3 (1)
(d) Dil HC1 (3) : Dil HNO3 (1)
Answer:
(c). is the correct answer.
Question 9.
Which of the following are not ionic compounds ?
(i) CaCl2
(ii) HCl
(iii) CCl4
(iv) NaCl
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iii)
Answer:
(b). Both HCl and CCl4 are covalent compounds in nature.
Question 10.
Which one of the following properties is not generally exhibited by ionic compounds ?
(a) Solubility in water
(b) Electrical conductivity in solid state
(c) High melting and boiling points
(d) Electrical conductivity in molten state.
Answer:
(b). Ionic compounds are conducting due to the movement of ions. Since the ions do not move in the solid state, these compounds are therefore, not conducting in the solid state.
Question 11.
Which of the following metals exist in their native state in nature ?
(i) Cu
(ii) Au
(iii) Zn
(iv) Ag
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer:
(c). Both Au and Ag are very little reactive. They exist in native state. However, the metal silver may also exist in some combined states (as minerals).
Question 12.
Metals are refined by using different methods. Which of the following metals are refined by electrolytic refining ?
(i) Ag
(ii) Cu
(iii) Na
(iv) Mg
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iii) and (iv)
Answer:
(a). Both Ag and Cu are very hard. These are called coinage metals and are purified by electrolytic refining.
Question 13.
Silver articles become black on prolonged exposure to air. This is due to the formation of
(a) AgCN
(b) Ag2O
(c) Ag2S
(d) Ag2S and AgCN
Answer:
(c). Air contains traces of hydrogen sulphide (H2S) gas which reacts with silver (Ag) to form Ag2S black in colour.
Question 14.
Galvanisation is a method of protecting iron from rusting by coating with a thin layer of
(a) Chromium
(b) Copper
(c) Zinc
(d) Tin
Answer:
(c). Coating the surface of iron with zinc is called galvanization.
Question 15.
Stainless steel is a very useful material for our life. In stainless steel, iron is mixed with
(a) Ni and Cr
(b) Cu and Cr
(c) Ni and Cu
(d) Cu and Au.
Answer:
(a). is the correct answer.
Question 16.
If copper is kept open in air, it slowly loses its shining brown surface and gains a green coating. It is due to the formation of :
(a) CuSO4
(b) CUCO3CO(OH)2
(c) CU(NO3)2
(d) CuO.
Answer:
(b). The green coating is due to the formation of basic copper carbonate which is CuCO3.Cu(OH)2.
Question 17.
Generally, metals are solid in nature. Which one of the following metals is found in liquid state at room temperature ?
(a) Na
(b) Fe
(c) Al
(d) Hg
Answer:
(d). Mercury (Hg) is a liquid at room temperature.
Question 18.
Which of the following metals are obtained by electrolysis of their chlorides in molten state ?
(i) Na
(ii) Ca
(iii) Fe
(iv) Cu
(a) (i) and (iv)
(b) (iii) and (iv)
(c) (i) and (iii)
(d) (i) and (ii)
Answer:
(d). Both Na and Ca are obtained by the electrolysis of their molten chlorides.
Question 19.
Generally non-metals are not lustrous. Which of the following non-metals is lustrous ?
(a) Sulphur
(b) Phosphorus
(c) Nitrogen
(d) Iodine.
Answer:
(d). Iodine is lustrous.
Question 20.
Which one of the following four metals would be displaced from the solution of its salts by other three metals ?
(a) Mg
(b) Cu
(c) Zn
(d) Fe
Answer:
(b). Cu is the least reactive and would be displaced from its salts by other metals.
Question 21.
5 mL each of concentrated HCl, HNO3 and a mixture of concentrated HCl (15 mL) and concentrated HNO3 (5 mL) were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C. The metal could be
(a) Al
(b) Au
(c) Cu
(d) Ag
Answer:
(b). Gold (Au) dissolves in aqua regia.
Question 22.
An alloy is :
(a) an element
(b) a compound
(c) a homogeneous mixture
(d) a heterogeneous mixture
Answer:
(c). An alloy is a homogeneous mixture of two or more elements (metals or non-metals.)
Question 23.
An electrolytic cell consists of :
(i) positively charged cathode
(ii) negatively charged anode
(iii) positively charged anode
(iv) negatively charged cathode
(a) (i) and (ii)
(b) (iii) and (iv)
(c) (i) and (iii)
(d) (ii) and (iv)
Answer:
(b).
Question 24.
During electrolytic refining of copper, ir gets
(a) deposited on cathode
(b) deposited on anode
(c) deposited on cathode as well as anode
(d) remains in the solution.
Answer:
(a). Pure metal is always deposited on cathode.
Question 25.
An element ‘X’ is soft and can be cut with a knife. This is very reactive to air and cannot be kept in open air. It reacts vigorously with water. Identify the element from the following
(a) Mg
(b) Na
(c) S
(d) Mg
Answer:
(b). Sodium (Na) gives all the characteristics of the element X.
Question 26.
Alloys are homogeneous mixtures of a metal with a metal or non-metal. Which among the following alloys contain non-metal as one of its constituents ?
(a) Brass
(b) Gun metal
(c) Amalgam
(d) Steel.
Answer:
(d). Steel (stainless) contains very small percentage of carbon.
Question 27.
Which of the following statements is not correct for magnesium metal ?
(a) It burns in oxygen with dazzling flame
(b) It reacts with cold water to form magnesium oxide and evolves hydrogen gas
(c) It reacts with hot water to form magnesium hydroxide and evolves hydrogen gas
(d) It reacts with steam to form magnesium hydroxide and evolves hydrogen gas.
Answer:
(b). Magnesium (Mg) does not react with cold water since its position in the reactivity series of metals is not at the top.
Question 28.
Which among the following alloys contain mercury as one of its constituents ?
(a) Stainless steel
(b) German silver
(c) Solder
(d) Zinc amalgam.
Answer:
(d). All amalgams contain mercury as one of their constituents.
Question 29.
Reaction between the elements X and Y results in the compound Z. Whereas X loses electron, Y gains the same. Which of the following properties is not shown by Z ?
(a) Has high melting point
(b) Has low melting point
(c) Conducts electricity in molten state
(d) Occurs as solid
Answer:
(b). Z is an ionic compound. Therefore, it is not expected to have low melting point.
Question 30.
The electronic configurations of three elements X, Y and Z are X — 2, 8; Y — 2, 8, 6 and Z — 2, 8, 1. Which of the following is correct ?
(a) X is a metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z is a metal.
Answer:
(d). Y is sulphur (S) while Z is sodium (Na)
Question 31.
Although metals form basic oxides, which of the following metals form an amphoteric oxide ?
(a) Na
(b) Ca
(c) Zn
(d) Cu.
Answer:
(c). Zn forms amphoteric oxide ZnO which reacts with both the acids and the alkalies.
Question 32.
Generally, non-metals are not conductors of electricity. Which of the following is a good conductor of electricity ?
(a) Diamond
(b) Graphite
(c) Phosphorus
(d) Iodine
Answer:
(b). Graphite is a good conductor of electricity.
Question 33.
Electrical wires have a coating of an insulating material. The material, generally used is
(a) Lead
(b) Graphite
(c) PVC
(d) All can be used.
Answer:
(c). PVC (Poly Vinyl Chloride) coatings are generally used for insulating electrical wires.
Question 34.
Which of the following non-metals is a liquid ?
(a) Carbon
(b) Bromine
(c) Iodine
(d) Sulphur
Answer:
(b). Non-metal bromine is a liquid but in the molecular form (Br2).
Question 35.
Which of the following can undergo a chemical reaction ?
(a) MgSO4 + Zn
(b) ZnSO4 + Fe
(c) CaSO4 + Pb
(d) CuSO4 + Al
Answer:
(d). Only Al can displace Cu from CuSO4 solution since it is placed above Cu in the activity series.
Question 36.
Which one of the following figures correctly describes the process of electrolytic refining ?
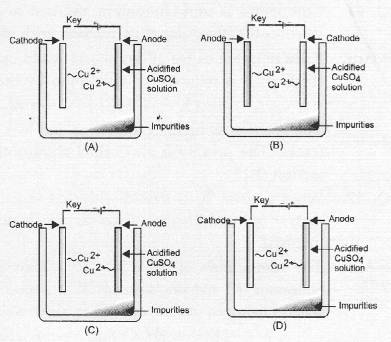
Answer:
(c). Cu2+ ions are released from anode by the oxidation of copper (Cu). These migrate towards cathode and are reduced to copper (Cu).
NCERT Exemplar Solutions for Class 10 Science Chapter 3 Short Answer Questions
Question 37.
Iqbal treated a lustrous, divalent element M with sodium hydroxide. He observed the formation of bubbles in reaction mixture. He made the same observations when this element was treated with hydrochloric acid. Suggest how can he identify the produced gas. Write chemical equations for both the reactions.
Answer:
The divalent element M is a metal. It reacts with both sodium hydroxide (alkali) and dilute hydrochloric acid to evolve hydrogen gas
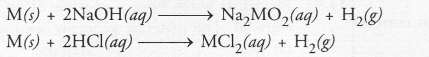
The gas burns with a pop sound when a burning match stick is brought near it.
Question 38.
During extraction of metals, electrolytic refining is used to obtain pure metals,
(a) Which material will be used as anode and cathode for refining of copper metal by this process
(b) Suggest a suitable electrolyte also,
(c) In this electrolytic cell, where do we get pure copper after passing electric current ?
Answer:
In the electrolytic refining of impure copper metal :
(a) A rod of impure copper is used as anode while that of pure copper as cathode.
(b) The electrolyte is a water soluble salt of copper. It is normally an aqueous solution of copper sulphate.
(c) On passing current, pure copper gets deposited at the cathode. It can be scrapped off later on. For more details.
Question 39.
Why should the metal sulphides and carbonates be converted to metal oxides in the process of extraction of metal from them ?
Answer:
It is quite easy to obtain a metal from metal oxide by carrying its reduction with a suitable reducing agent. However, metal sulphides and carbonates are converted to the oxide form and then reduced.
Question 40.
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), are treated with HNCfy, hydrogen is not liberated, why ?
Answer:
HNO3 acts as a powerful oxidising agent along with acting as acid. In this case, hydrogen gas which is intially involved is oxidised to water.
Question 41.
Compound A’ and aluminium are used to join railway tracks,
(a) Identify the compound A’
(b) Name the reaction
(c) Write down its reaction with aluminium.
Answer:
Railway trakcs as we all know, are made up of iron. This means that the compound A is an oxide of iron (Fe2CO3). It is reduced by aluminium by thermit reaction with aluminium.
![]()
The reaction is highly exothermic and as a result, iron is in molten state and can weld the broken railway tracks. The process is also called aluminothermie reduction or thermit process.
Question 42.
When a metal ‘X’ is treated with cold water, it gives a basic salt ‘Y’ with molecular formula XOH (Molecular mass = 56) and liberates a gas ‘Z’ which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Answer:
The atomic mass of metal X = 56 – Mass of OH group = 56 — 17 = 39u. This shows that the metal ‘X’ is potassium (K) and the basic salt ‘Y’ is potassium hydroxide (KOH). It is formed by reacting potassium with cold water. Hydrogen gas ‘Z’ evolved in the reaction catches fire.
![]()
Question 43.
A non-metal X-exists in two different forms Y and Z. Y is the hardest natural substance, whereas Z is a good conductor of electricity. Identify X, Y and Z.
Answer:
Since “Y” is the hardest occuring non-metal, it is diamond. The non-metal “X” is carbon. Y is the crystalline allotropie form of carbon. The other allotropie form Z which is a good conductor of electricity is graphite.
Question 44.
The following reaction takes place when aluminium powder is heated with Mn02.
![]()
(a) Is aluminium getting reduced ?
(b) Is Mn02 getting oxidised ?
Answer:
(a) Aluminium is getting oxidised to Al2O3.
(b) Manganese dioxide is getting reduced to Mn.
Question 45.
What are the constituents of solder alloy ? Which property of solder makes it suitable for welding electrical wires ?
Answer:
The constituents of solder alloy are ; lead (Pb) and tin (Sn). Being a low melting solid and also a good conductor of electricity, solder is used for welding electrical wires and cables.
Question 46.
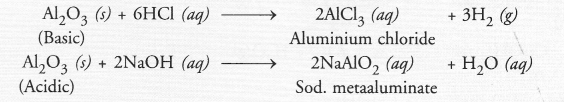
A metal ‘M’, which is used in thermite process, when heated with oxygen gives an oxide, which is amphoteric in nature. Identify ‘M’ and its oxide. Write down the reactions of oxide with HCl and NaOH.
Answer:
The information suggests that the metal ‘M’ is aluminium (Al). The formula of its oxide is Al2O3 which is amphoteric in nature. It reacts with both acid and base.
Question 47.
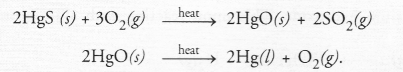
A metal that exists as a liquid at room temperature is obtained by heating its sulphide in the presence of air. Identify the metal and its ore and give the reaction involved.
Answer:
Mercury is the metal which exists as a liquid at room temper nature. It can be obtained by heating the sulphide ore in the presence of air. The process is known as roasting. The chemical reactions involved are :
Question 48.
Give the formulae of the stable binary compounds that would be formed by the combination of following pairs of elements.
(a) Ca and N2
(b) Li and O2
(c) Ca and Cl2
(d) K and
Answer:
(a) Calcium nitride (Ca3N2)
(b) Lithium oxide (Li2O)
(c) Calcium chloride (CaCl2)
(d) Potassium oxide (K2O).
Question 49.
What happens when
(a) ZnCO3 is heated in the absence of oxygen ?
(b) a mixture of Cu2O and Cu2S is heated ?
Answer:
(a) A mixture of ZnO(s) and CO2 is formed

(b) A mixture of copper and SO2 is formed.

Question 50.
A non-metal A is an important constituent of our food and forms two oxides B and C. Oxide B is toxic whereas C causes global warming
(a) Identify A, B and C
(b) To which Group of Periodic Table does A belong ?
Answer:
The non-metal A is carbon. It is an important constituent of our food in different forms. For example, glucose (C6H12O6) contains carbon. In fact, all food materials are organic compounds and these contain carbon as essential constituent. The two oxides of carbon are, carbon monoxide (B) and carbon dioxide (C). Carbon dioxide causes global warming.
(a) A = Carbon (C) ;
B = Carbon monoxide (CO) ;
C = Carbon dioxide (CO2)
(b) Carbon is the first member of group 14 in the Long form of Periodic Table.
Question 51.
Give two examples each of the metals that are good conductors and poor conductors of heat respectively.
Answer:
Good conductors of heat : Ag and Cu Poor conductors of heat : Pb and Hg.
Question 52.
Name one metal and one non-metal that exist in liquid state at room temperature. Also name two metals having melting point less than 310 K (37°C).
Answer:
The metal mercury (Hg) and non-metal (Br2) exist in liquid state at room temperature.
The two metals with melting points less than 310 K are ; cesium (Cs) with melting point 301 K and gallium (Ga) with melting point 303 K.
Question 53.
An element ‘A’ reacts with water to form a compound ‘B’ which is used in white washing. The compound ‘B’ on heating forms an oxide ‘C’ which on treatment with water gives back ‘B’. Identify ‘A’, ‘B’ and ‘C’ and give the reactions involved.
Answer:
Since the compound ‘B’ is used for white wash, it is calcium hydroxide and the element ‘A’ is calcium. Upon heating ‘B’ forms calcium oxide ‘C’. It reacts with water to give calcium hydroxide ‘B’ again.
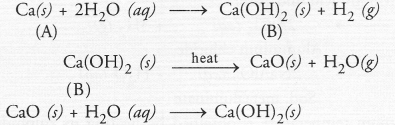
Question 54.
An alkali metal A gives a compound B (molecular mass = 56) on reacting with water. The compound ‘B’ gives a soluble compound ‘C’ on treatment with aluminium oxide. Identify A, B and C and give the reaction involved.
Answer:
As explained under Question 42, the alkali metal A is potassium (K) and on reacting with water, it forms a compound ‘B’ which is potassium hydroxide. This upon reacting with aluminium oxide (Al2O3) forms potassium metaluminate ‘C’.
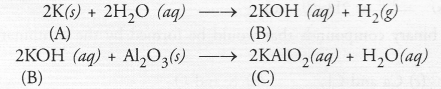
Question 55.
Give the reaction involved during extraction of zinc from its ore by
(a) roasting of zinc ore
(b) calcination of zinc ore
Answer:
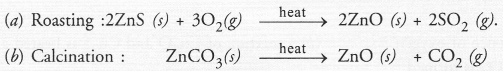
Question 56.
A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black coloured product. Identify M and black coloured product and also explain the reaction of M with oxygen.
Answer:
The avilable information suggests that the metal M is copper (Cu). It does not liberate hydrogen since it is placed below hydrogen in the reactivity series. Copper reacts with oxygen upon heating to form cupric oxide (CuO) which is black in colour.
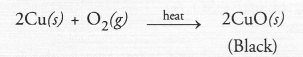
Question 57.
An element forms an oxide A2O3 which is acidic in nature. Identify A as a metal or non-metal.
Answer:
Since the oxide A2O3 is acidic in nature, the element A is non-metal.
Question 58.
A solution of CuSO4 was kept in an iron pot. After few days, the iron pot was found to have a number of holes in it. Explain the reason in terms of reactivity. Write the equation of the reaction involved.
Answer:
Iron (Fe) is placed above copper (Cu) in the reactivity series. This means that a chemical reaction had occurred between iron (iron pot) and aqueous CuSO4 solution.
![]()
Since iron was consumed in the reaction, a number of holes appeared in the pot.
NCERT Exemplar Solutions for Class 10 Science Chapter 3 Long Answer Questions
Question 59.
A non-metal A which is the largest constituent of air, when heated with H2 in 1 : 3 ratio in the presence of catalyst (Fe) gives a gas B. On heating with O2 it gives an oxide C. If this oxide is passed into water in the presence of air, it gives an acid D which acts as a strong oxidising agent.
(a) Identify A, B, C and D.
(b) To which group of periodic table does this non-metal belong ?
Answer:
(a) The available information suggests that the non-metal A is nitrogen (N) and its molecular form is N2. It is the major constituent of air (about 79 per cent by volume). It reacts with H2 upon heating m the presence of iron catalyst (Fe) to form ammonia (NH3) gas(g)
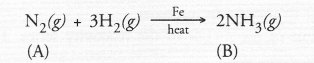
Upon heating with oxygen, nitrogen forms intially nitric oxide (NO) and then nitrogen dioxide (C). The latter reacts with water in the presence of oxygen to form nitric acid (D). The acid acts as strong oxidising agent.
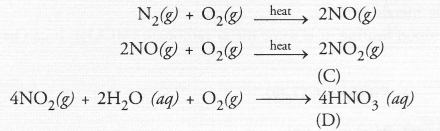
(b) The element nitrogen is the first member of group 15 of Modern periodic table.
Question 60.
Give the steps involved in the extraction of metals of low and medium reactivity from their respective sulphide ores.
Answer:
Extraction of Metals present low in the Activity series
Silver (Ag), gold (Au) and platinum (Pt) generally occur in the free or native state. This means that they can be isolated rather easily. Metals like copper (Cu) and mercury (Hg) are comparitively more reactive and occur in combined states. For example, the ore of mercury is cinnabar (HgS) while that of copper is copper glance (Cu2S). Both are converted into metallic form upon heating in air or oxygen.
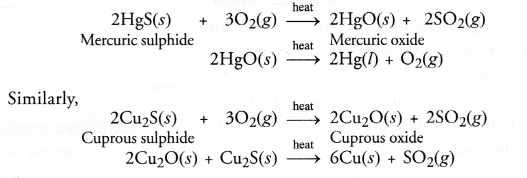
Extraction of Metals present in the middle of the Activity series
The metals present in the middle of the series are zinc, iron, chromium, nickel, cobalt, lead etc. These are usually present as sulphides or carbonares in nature. However, it is quite easy to obtain a metal from its oxide form which is then reduced to the metallic state. The various steps involved in the process of extraction are briefly discussed.
(a) Calcination :
Calcination may be defined as the process of heating the ore below its melting point in the absence of air.
As a result of calcination, the following changes take place
- Moisture is driven out and the ore becomes dry.
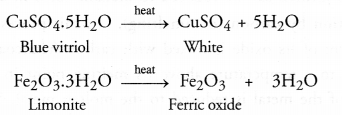
- Some hydrated ores decompose and become anhydrous by losing molecules of water of crystallisation. For example, heat
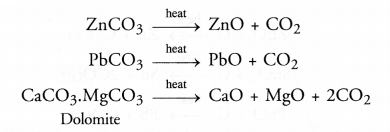
(b) Roasting :
Roasting may be defined as the process of heating the ore below its melting point with excess of air.
As a result of roasting, the following changes occur :
- Any organic matter if present, gets destroyed.
- Impurities of non-metals such as sulphur, arsenic or phosphorus are converted into their volatile oxides which are removed. For example,
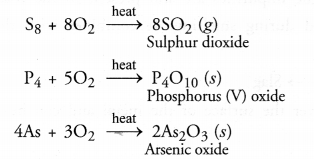
- Sulphides of the metals are converted into their oxides. For example,
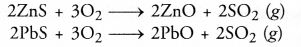
- Small amounts of sulphides may also be converted into sulphates as as result of roasting. For example,
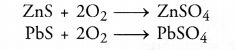
Question 61.
Explain the following
(a) Reactivity of Al decreases if it is dipped in cone. HNO3
(b) Carbon cannot reduce the oxides of Na or Mg.
(c) NaCl is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as in molten state
(d) Iron articles are galvanised.
(e) Metals like Na, K, Ca and Mg are never found in their free state in nature.
Answer:
(a) When Al metal is dipped in cone. HNO3 for sometime, it is oxidised initially to aluminium oxide (Al2O3). The oxide gets deposited on the surface of the metal and forms a protective coating an the surface. The metal is said to become passive towards air, acids and alkalies. Its reactivity therefore, decreases.
(b) Both Na and Mg are more reactive than carbon. Therefore, carbon is not in a position to reduce the oxides of these metals.
(c) NaCl is an ionic compound. Its electrical conductivity is due to the mobility of Na+ and Cl– ions. These ions cannot move in the solid state. However, they can do so either in molten state of the salt or when it forms and aqueous solution in water.
(d) Iron has a tendency to get rusted in atmosphere by reacting with oxygen and water vapours present in air. In order to check rusting, iron articles are generally coated with zinc. This process is known as galvanization.
(e) All these metals are placed high in reactivity series of metals. They are quite reactive and do not exist in free states.
Question 62.
(i) Given below are the steps lor extraction of copper from its ore. Write the reaction involved.
(a) Roasting of copper (1) sulphide
(b) Reduction of copper (1) oxide with copper (1) sulphide.
(c) Electrolytic refining
(ii) Draw a neat and well labelled diagram for electrolytic refining of copper.
Answer:
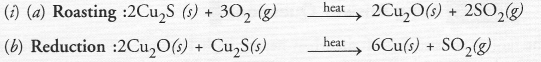
This reaction in which one of the reactants (Cu2S) carries the reduction of the product (Cu2O) is known as auto-reduction.
(c) Reactions taking place in electrorefensing are :
![]()
(ii) For the diagram of electro-refining,
Question 63.
Of the three metals X, Y and Z, X reacts with cold water, Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in order of increasing reactivity.
Answer:
The answer is based on the relative positions of the metals in the reactivity series. The reactivity with water decreases down the series.
- Metal X is Na or K
- Metal Y is Mg or Ca
- Metal Z is Al or Fe.
Order of increasing reactivity : Z < Y < X.
Question 64.
Two ores ‘X’ and ‘Y’ were taken. On heating, ore ‘X’ gives CO2 whereas ore ‘Y’ gives SO2. What steps will you take to convert them into respective metals ?
Answer:
Since the ore ‘A’ of the metal gives CO2 upon heating, it is some metal carbonate (MCO3). It can be converted to the metallic form as follows :
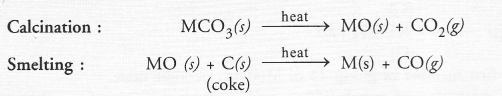
Since the ore ‘Y’ of the metal gives SO2 upon heating, it can be some metal sulphide (MS). It can be converted to the metallic form as follows :
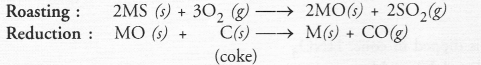
NCERT Exemplar Class 10 Science Chapter 3 Metals And Non-Metals
Short Answer Type Questions
1.Iqbal treated a lustrous, divalent element M with sodium hydroxide. He observed the formation of bubbles in reaction mixture. He made the same observations when this element was treated with hydrochloric acid. Suggest how can he identify the produced gas. Write chemical equations for both the reactions.
Answer:
M + 2NaOH —>Na2M02 + H2(g)
M + 2HCl —> MCl2 + H2(g)
Bring a burning candle near the gas. If it burns with pop sound, the gas is hydrogen and the element is a metal.
2 During extraction of metals, electolytic refining is used to obtain pure metals.
(a) Which material will be used as anode and cathode for refining of silver metal by this process?
(b) Suggest a suitable electrolyte also.
(c) In this electrolytic cell, where do we get pure silver after passing electric current?
Answer:
(a) Pure silver rod will be used as cathode and impure silver rod will be used as anode.
(b) Ag NO3 (aq) can be used as electrolyte.
(c) Pure silver will be formed at cathode.
At anode : Ag —> Ag+ +e–
At cathode : Ag+ +e–—> Ag
3.Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNOs, hydrogen is not liberated. Why?
Answer.It is because HNO3 is an oxidising agent and it gets reduced to NO if it is dilute and NO2if HNOs is concentrated, it oxidises H2 to H2O.
4.Compound X and aluminium are used to join railway tracks.
(a) Identify the compound X.
(b) Name the reaction.
(c) Write down its reaction.
Answer.

5.What are the constituents of solder alloy? Which property of solder makes it suitable for welding electrical wires?
Answer. Solder is made up of lead and tin. It has low melting point, therefore, it is used for soldering (welding) electrical wires.
6.A metal A, which is used in thermite process, when heated with oxygen gives
an oxide B, which is amphoteric in nature? Identify A and B. Write down the reactions of oxide B with HC1 and NaOH.
Answer: ‘A’ is aluminium.

7.What happens when
(a) ZnCOs is heated in the absence of oxygen?
(b) a mixture of Cu2O and Cu2S is heated?
Answer:

8.A non-metal A is an important constituent of our food and forms two oxides B and C. Oxide B is toxic whereas oxide C causes global warming.
(a) Identify A, B and C.
(b) To which group of periodic table does A belong?
Answer:
(a) A is carbon. It forms two oxides: CO (B) is toxic whereas CO2 (C) causes
global warming as it absorbs heat radiations from atmosphere.
(b) A belongs to group 14 of periodic table.
9.An element A reacts with water to form a compound B which is used in white washing. The compound B on heating forms an oxide C which on treatment with water gives back B. Identify A, B and C and give the reactions involved.
‘A’ is calcium metal, ‘B’ is calcium hydroxide, ‘C’ is calcium oxide.
Answer:

10. A metal M does not liberate hydrogen from acids but reacts reacts with oxygen to give a black colour product. Identify M and black coloured product and also explain the reaction of M with oxygen.
Answer:
M is ‘Cu’. It does not liberate hydrogen with dilute acid as it is less reactive than hydrogen gas.

11. A solution of CuS04 was kept in an iron pot. After few days, the iron pot was found to have a number of holes in it. Explain the reason in terms of reactivity. Write the equation of the reaction involved.
Answer: Iron is more reactive than copper. Therefore, it displaces copper from copper sulphate solution.

12. When a metal ‘X’ is treated with cold water, It gives base salt ‘X’ with molecular formula XOH (Molecular mass = 40) and liberate a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Answer.
‘X’ is Na ‘Y’ is NaOH ‘Z’ is H2 gas

13. A non-metal ‘X’ exists in two different forms ‘Y’ and ‘Z\ ‘Y’ is hardest natural substance, whereas ‘Z’ is good conductor of electricity. Identify X, Y and Z.
Answer: ‘X’ is carbon ‘Y’ is diamond ‘Z’ is graphite.
‘Y’ (diamond) is hardest natural substance.
‘Z’ (graphite) is good conductor of electricity.
14. The following reaction takes place when aluminium powder is heated with MnO2 :
3MnO2(s) + 4Al(s)—–> 3Mn(l) + 2Al2O3(l) + Heat
Molten Molten
(a) Is aluminium getting reduced ?
(b) Is MnO2 getting oxidised ?
Answer:
(a) No, aluminium is getting oxidised.
(b) No, MnO2 is getting reduced.
15. A metal that exists as liquid at room temperature is obtained by heating its sulphide in the presence of air. Identify the metal and its ore and give the reaction involved.
Answer: The metal is mercury.
2HgS(s) + 3O2(g)—–> 2HgO(s) + 2SO2(g)
HgS(s) + 2HgO(s)—–> 3Hg(l) + SO2(g)
16. Give the formulae of the stable binary compounds that would be formed by the combination of following pairs of elements :
(a) Mg and N2 (b) Li and 02
(c) A1 and Cl2 (d) K and O2
Answer:
(a) 3Mg + N2—–> Mg3N2
(b) 4Li + O2—–> 2Li20
(c) 2Al + 3Cl2—–> 2AlCl3
(d) 4K + O2 —–> 2K2O
17.Give two examples each of the metals that are good conductors and poor conductors of heat respectively.
Answer: Good conductors of heat are copper and silver. Poor conductors of heat are lead and mercury.
18. Name-one metal and one non-metal that exist in liquid state at room temperature. Also name two metals having melting point less than 310 K (37 °C).
Answer: Metal in liquid state is mercury, non-metal in liquid state is bromine.
Gallium (Ga) and Cesium (Cs) have melting point below 310 K.
19. An alkali metal A gives a compound B (molecular mass = 40) on reacting with water. The compound B gives a soluble compound C on treatment with aluminium oxide. Identify A, B and C and give the reaction involved.
Answer:

‘C’ is sodium meta aluminate, soluble in water.
20. Give the reaction involved during extraction of zinc from its ore by
(a) roasting of zinc ore.
(b) calcination of zinc ore.
Answer:

21. An element forms an oxide, A2Os which is acidic in nature. Identify A as a metal or non-metal.
Answer: A is non-metal as non-metallic oxides are acidic in nature.
Long Answer Type Questions
22. A non-metal A which is the largest constituent of air, when heated with H2 in 1 : 3 ratio in the presence of catalyst (Fe) gives a gas B. On heating with O2, it gives an oxide C. If this oxide is passed into water in the presence of air, it gives an acid D which acts as a strong oxidising agent.
(a) Identify A, B, C and D.
(b) To which group of periodic table does this non-metal belong?
Answer:
(a) N2 is largest constituent of air, when heated with H2 in the ratio of 1 : 3 .
in the presence of catalyst (Fe) gives a gas NH3(B).

Nitrogen reacts with oxygen on heating to form nitrogen monoxide ‘C’, which gets oxidised in the presence of O2 to nitrogen dioxide. Nitrogen dioxide dissolves in water in the presence of oxygen to form nitric acid which is an oxidising agent.


‘A’ is N2, ‘B’ is NH3, ‘C’ is NO and ‘D’ is HNO3.
(b) Group 15
23. Explain the following:
(a) Reactivity of A1 decreases if it is dipped in HNO3.
(b) Carbon cannot reduce the oxides of Na or Mg.
(c) NaCl is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state.
(d) Iron articles are galvanised.
(e) Metals like Na, K, Ca and Mg are never found in their free state in nature.
Answer:
(a) It is due to the formation of oxide layer on its surface.
(b) It is because Na or Mg are strong reducing agents, therefore, carbon cannot reduce their oxides.
(c) NaCl does not conduct electricity in solid state as it does not have free ions to move in solid state but in aqueous solution and in molten states ions are free to move.
(d) Iron articles are galvanised to protect them from rusting.
(e) It is because these metals are highly reactive and occur in the form of their compounds.
24.An element A burns with golden flame in air. It reacts with another element B, atomic number 17 to give a product C. An aqueous solution of product C on electrolysis gives a compound D and liberates hydrogen. Identify A, B, C and D. Also write down the equations for the reactions involved, HOTS;
Answer: A is sodium as it burns with golden flame in air.
‘B’ (17) has electronic configuration 2, 8, 7 so ‘B’ is chlorine

A is Na, ‘B’ is Cl2 , ‘C’ is NaCl and ‘D’ is NaOH.
25.Give the steps involved in the extraction of metals of low and medium reactivity from their respective sulphide ores.
Answer:

Metal sulphide like HgS reacts with 02 to form mercuric oxide and sulphur dioxide. Mercuric oxide is unstable changes to Hg(l) which can be refined by distillation.
2HgS 4- 3O2 —–> 2HgO + 2SO2
HgS + 2HgO —–> 3Hg + SO2
Sulphide ore like Zn, on roasting (in presence of O2 ) gives metal oxide which on reduction with metal sulphide (ZnS) or suitable reducing agent gives metal which is refined by electrolyte refining.
2ZnS + 3O2 —–> 2ZnO + 2SO2
2ZnO + ZnS —–> 3Zn + SO2
Alternative method of reduction :
ZnO + C —–> Zn + CO
Pure zinc is obtained by electrolytic refining.
26.Of the three metals X, Y and Z, X reacts with cold water, Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in order of increasing reactivity.
Answer: X’ is sodium or potassium metal.
2Na(s) + 2HsO(l) —–> 2NaOH(aq) + H2(g)
‘X’ ‘cold’
Or
2K(s) + 2H2O(l) —–> 2KOH(aq) + H2(g)
‘X’ ‘cold’

Fe < Mg < Na < K is an increasing order of reactivity.
27.Two ores A and B were taken. On heating, ore A gives CO2 whereas, ore B gives SO2 . What steps will you take to convert them into metals ?
Answer:Since ore ‘A’ gives CO2 and ore ‘B’ gives SO2 . Therefore, A is metal carbonate ore and ‘B’ is metal sulphide ore.
Metal carbonate on heating gives ‘MO’.

‘MO’, on reduction with coke gives metal and carbon monoxide.
![]()
Metal ‘M’ is obtained which can be purified by electrolytic refining.
Metal sulphide is heated in presence of oxygen to form metal oxide and S02.

Metal sulphide reacts with metal oxide to form impure metal and S02.
![]()
MO can also be reduced with carbon to form metal. The metal ‘M’ from ore ‘B’ is obtained.
![]()
28.Impure metal is purified by electrolytic refining
(i) Given below are steps for extraction of copper from its ore. Write the reaction involved.
(a) Roasting of copper (I) sulphide
(b) Reduction of copper (I) oxide with copper (I) sulphide
(c) Electrolytic refining
(ii) Draw the neat and labelled diagram for electrolytic refining of copper.
Answer:
(i)
(a) 2CU2S(S) + 3O2(g) ——–> 2Cu2O(s) + 2SO2(g)
(b) 2CU2O(S) + Cu2S(s) ——–> 6CU(s) + SO2(g)

