NCERT Solutions for Class 10 Science Chapter 5 Periodic Classification of Elements
NCERT Solutions for Class 10 Science Chapter 5 Periodic Classification of Elements
Periodic Classification of Elements Class 10 Science In Text Book Questions
Question 1.
Did Doberiener’s triads also exist in the columns of Newlands’ octaves ? Compare and find out.
Answer:
Yes, some of the Doberiener’s triads did exist in the columns of Newlands’ octaves. For example,
![]()
Question 2.
What were the limitations of Doberiener’s triads ?
Answer:
Doberiener was in a position to identify three triads. It could not apply to all the elements known at that time. Therefore, the classification was not so useful.
Question 3.
What were the limitations of Nawlands’ Law of Octaves ?
Answer:
- Actually this classification was successful only upto the element calcium. After that, every eighth element did not possess the same properties as by the element lying above it in the same group. For example, the elements cobalt (Co) and nickel (Ni) placed below chlorine had different properties. Same was the case with copper (Cu) placed after potassium in the same group.
- Newland committed another mistake. He placed two elements in the same slot in a particular group. For example, Co and Ni in the first group after chlorine. Similarly, elements cerium (Ce) and lanthanum (La) were placed after yitterium (Y) in the same group. Newland could not offer any explanation for such an arrangement.
- Newland somehow thought that only 56 elements existed in nature and no more elements were likely to be discovered. But this belief ultimately proved to be wrong.
- When noble gas elements were discovered at a later stage, their inclusion in the table disturbed the entire arrangement.
Question 4.
Use Mendeleev’s periodic table to predict the formulae for the oxides of the elements : K, C, Al, Si, Ba.
Answer:
Oxygen is a member of group VTA in Mendeleev’s periodic table. Its valency is 2. Similarly, the valencies of all the elements listed can be predicted from their respective groups. This can help in writing the formulae of their oxides.
- Potassium (K) is a member of group IA. Its valency is 1. Therefore, the formula of its oxide is K2O.
- Carbon (C) is a member of group IVA. Its valency is 4. Therefore, the formula of its oxide is C2O4 or CO2.
- Aluminium (Al) belongs to groups IIIA and its valency is 3. The formula of the oxide of the element is Al2O3.
- Silicon (Si) is present in group IVA after carbon. Its valency is also 4. The formula of its oxide is Si2O4 or SiO2
- Barium (Ba) belongs to group IIA and the valency of the element is 2. The formula of the oxide of the element is Ba2O2 or BaO.
Question 5.
Besides gallium which two other elements have since been discovered that fill the gaps left by Mendeleev in creating his periodic table ?
Answer:
Two other elements are scandium (Sc) and germanium (Ge). In their gaps, the elements with names Eka-boron and Eka-silicon were placed.
Question 6.
What was the criteria used by Mendeleev in creating his periodic table ?
Answer:
Mendeleev used atomic masses of the elements as the criteria for creating his periodic table. In this table, the elements were arranged in order of increasing atomic masses.
Question 7.
Why do you think that the noble gases should be placed in a separate group ?
Answer:
In the Mendeleev’s periodic table, the elements have been arranged in the different groups on the basis of valency. For example, the elements placed in group I (IA and IB) have Valency equal to one. Same is the
case with the elements placed in other groups. Since the noble gas elements He, Ne, Ar, Kr, Xe, and Rn have zero valency, they could not find a place in Mendeleev’s periodic table. These have been placed in a separate group called zero group in the periodic table. Please note that the noble gas elements were not a part of the Mendeleev’s periodic table. They were added later on.
Question 8.
How could the Modern Periodic table remove various anomalies of Mendeleev’s periodic table ?
Answer:
We have already studied that the Mendeleev’s periodic table is based on the atomic masses of the elements whereas Modern Periodic Table takes into account their atomic numbers. Since the properties of the elements are linked with the electronic configuration of their atoms (i.e., atomic number), this means that Modern Periodic Table is better than the Mendeleev’s Periodic Table. The important advantages are listed.
- The position of the elements in the periodic table are linked with their electronic configuration.
- Each group is an independent group and the idea of sub-groups has been discarded.
- One position for all the isotopes of an element is justified since the isotopes have the same atomic number. For example, the three isotopes of the element hydrogen e., protium, deuterium and tritium have atomic number one (Z = 1). Similarly, two isotopes of chlorine i.e. Cl-35 and Cl-37 are placed in the same slot since they have same atomic number (Z = 17).
- The positions of certain elements which were earlier misfits in the Mendeleev’s periodic table are now justified because it is based on atomic numbers of the elements. For example, Ar precedes K because its atomic number (18) is less than that of K (19).
- It is quite easy to remember and reproduce.
Question 9.
Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice ?
Answer:
Magnesium (Mg) belongs to group 2 known as Alkaline Earth Family. The two other elements belonging to the same group are calcium (Ca) and strontium (Sr). The basis of choice is the electronic distribution in the valence shell of these elements. All of them have two electrons each.
| K | L | M | N | O | |
| Mg (Z =12) | 2 | 8 | 2 | — | — |
| Ca (Z = 20) | 2 | 8 | 8 | 2 | — |
| Sr (Z = 38) | 2 | 8 | 18 | 8 | 2 |
Question 10.
Name :
(a) three elements that have a single electron in their outermost shells.
(b) three elements that have two electrons in their outermost shells.
(c) three elements with filled outermost shells.
Answer:
(a) Lithium, sodium, potassium (Alkali metals present in group 1)
(b) Beryllium, magnesium, calcium (Alkaline earth metals present in group 2)
(c) Helium, neon, argon (Noble gases present in group 18).
Question 11.
(a) Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements ?
(b) Helium is an unreactive gas and neon is a gas of extremely low reactivity. What, if anything, do their atoms have in common ?
Answer:
(a) The atoms of all these elements have one electron each in their valence shell. That is why, these elements are placed in the group 1 known as alkali metal group. The electronic configurations of these elements are given :
| K | L | M | N | |
| Li (Z = 3) | 2 | 1 | — | — |
| Na (Z = 11) | 2 | 8 | 1 | — |
| K (Z = 19) | 2 | 8 | 8 | 1 |
All the three elements evolve hydrogen gas on reacting with water
2 Li + 2 H2O ———-> 2 LiOH + H2
2 Na + 2 H2O ————> 2 NaOH+ H2
2 K + 2 H2O ————> 2 KOH + H2
Apart from this, all the elements happen to be the first elements of their respective periods. For example,
- Second period starts from lithium (Li)
- Third period starts from sodium (Na)
- Fourth period starts from potassium (K).
(b) Both these elements have completely filled shells. Helium (Z = 2) has two electrons in the only shell (K shell). The other element neon (Z = 10) has both K and L shells fully filled (2, 8). Because of the filled shells, the atoms of these elements do not have any desire to take part in chemical combination and they have been placed together in the same group known as group 18 or zero group.
Question 12.
In the modern periodic table, which are the metals among the first ten elements ?
Answer:
Metals among the first ten elements are lithium (Li) and beryllium (Be) . These are placed towards the left of the table.
| Period | Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 |
| 1
2 |
—
— |
—
Be |
—
— |
—
— |
—
— |
—
— |
| 3
4 |
—
— |
—
— |
—
Ga |
—
Ge |
—
As |
—
Se |
Question 13.
By considering their position in the periodic table, which one of the following elements would you expect to have maximum metallic characteristics ?
Answer:
Before identifying the metallic character from the list of the elements, we must remember two points :
- The metallic character of an element is related to the electron releasing tendency of its atoms. Greater the tendency, more will be the metallic character.
- In general, metallic character of the elements increases down the group and decreases along a period.
With the help of the Modern Periodic Table, let us identify the group and period to which these elements
Since the metallic character increases down a group and decreases along a period, the obvious choice is between two elements. These are Be (beryllium) present in group 2 and Ga (gallium) present in group 13. Now, the size of Ga is very big as compared to that of Be. Actually, the element Ga has three shells since it belongs to period 4 while the element Be has only two shells as it is a member of period 2. This means that the element Ge has a greater electron releasing tendency of its atom as compared to the element Be. Therefore, Ge has more metallic character, rather maximum metallic character among the elements that are listed.
Periodic Classification of Elements Class 10 Science NCERT End Exercise
Question 1.
Which of the following statements is not correct about the trends while going from left to right across the periodic table ?
(a) The elements become less metallic in nature
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
Answer:
(c). The atoms lose their valence electrons with difficulty and not easily. This is on account of the reason that
- nuclear charge increases from left to the right since the atomic number of the elements gradually increases.
- with the increase in nuclear charge, the force binding the electrons with the nucleus increases. Therefore, the atoms lose their valence electrons with difficulty.
Question 2.
Element X forms a chloride with the formula XCI2 which is a solid with high melting point. X would most likely to be in the same group of the periodic table as :
(a) Na
(b) Mg
(c) Al
(d) Si.
Answer:
(b). The formula of the chloride of the element is XCl2. This means that the valency of the element X is 2 since chlorine is monovalent. The element with valency 2 is expected to be present in group 2 to which magnesium (Mg) belongs.
Question 3.
Which element has :
(a) two shells, both of which are completely filled with electrons ?
(b) the electronic configuration 2, 8, 2 ?
(c) a total of three shells with four electrons in the valence shell ?
(d) a total of two shells with three electrons in the valence shell ?
(e) twice as many electrons in the second shell as in the first shell ?
Answer:
(a) The elements with completely filled shells are noble gas elements and they belong to group 18. Since the element has two shells ; it must be present in second period and is neon (Ne) with electronic configuration 2, 8.
(b) The electronic configuration suggests that the element belongs to third period and second group. It is therefore, magnesium (Mg).
(c) The element with three shells is present in third- period. Since it* has four electrons in the valence
shell, it must belong to group 14 and is silicon (Si) with electronic configuration 2, 8, 4.
(d) The element with two shells is expected to be present in the second period. With three electrons in
the valence shell, it must belong to group 13 and is boron (B) with electronic configuration 2, 3.
(e) The element has only two shells. The first shell can have a maximum of two electrons. The second shell has four electrons which is twice the number of electrons present in the first shell. Therefore, the electronic configuration of element is 2, 4. It is carbon with atomic number (Z) equal to 6.
Question 4.
(a) Which property do all elements in the same column of the periodic table as boron have in common ?
(b) Which property do all elements in the same column of the periodic table as fluorine have in common ?
Answer:
(a) The element boron (B) is the first member of group (also called column) 13. It has three electrons in
the valence shell (2, 3). The other elements included in the same column are aluminium (Al),
gallium (Ga), indium (In) and thalium (Tl). They too have three electrons in the valence shell of their atoms. Just like boron, these elements also show a valency of 3 in their compounds.
(b) The element fluorine (F) is the first member of group (also called column) 17. It has seven electrons in the valence shell (2, 7). The other members present in the same group known as halogen family are chlorine (Cl), bromine (Br), iodine (I) and astatine (At). They have also seven electrons in the valence shell of their atoms. Like fluorine, they all show a valency of 1 in their compounds.
Question 5.
An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element ?
(b) To which of the following elements would it be chemically similar ? (Atomic numbers are given in parentheses).
N(7), F(9), P(15), Ar(18).
Answer:
(a) The atomic number of the element is 17 (2 + 8 + 7 = 17).
(b) It would be chemically similar with fluorine (F) which has also 7 electrons in valence shell (2, 7)
Question 6.
The position of three elements A, B and C in the periodic table are shown below :
| Group 16 | Group 17 |
| – | A |
| B | C |
(a) State whether A is metal or non-metal.
(b) State whether C is more reactive or less reactive than A.
(c) Will C be larger or smaller in size than B ?
(d) Which type of ion, cation or anion will be formed by the element A ?
Answer:
(a) Group 17 represents halogen family. All the elements included in the family are Therefore, element A is a non-metal.
(b) Reactivity of non-metals is generally due to the electron accepting tendency of their atoms. Down the group, the atomic size increases. Therefore, the attraction of the nucleus for the outside electrons decreases. This means that down the group of non-metals, reactivity decreases. Thus, the element C is less reactive than the element A.
(c) Atomic size of the elements decreases along a period. The elements B and C are present in the same period. Since C is placed after B, the size of the element C is less than that of B.
(d) The elements A, as pointed out earlier is a non-metal which belongs to group 17. It has seven valence electrons (2, 8, 7). In order to have the configuration of the nearest noble gas element, it will take up one electron and change to anion i.e., A– ion.
Question 7.
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the periodic table. Write their electronic configuration. Which of these will be more electronegative and why ? (CBSE 2012)
Answer:
The electronic configurations of the two elements are :
Nitrogen (Z = 7) 2, 5 ;
Phosphorus (Z = 15) 2, 8, 5
Since the size of nitrogen is small as compared to phosphorus, it has a greater tendency to take up electrons. It is therefore, more electronegative than phosphorus.
Question 8.
How does the electronic configuration of an atom relate to its position in the modern periodic table ?
(CBSE Delhi 2011)
Answer:
The modern periodic table is based upon atomic numbers of the elements. Since electronic configurations of the elements depend upon their atomic numbers, this means that the periodic table is based on the electronic configurations of the elements. For example, all the alkali metals have one electron each in their valence shell. These are placed in group 1. Similarly, the alkaline earth metals with two electrons in their valence shell are placed in group 2 and so on.
Question 9.
In the modern periodic table, calcium (Z = 20) is surrounded by the elements with atomic numbers 12, 19, 21 and 38. Which of these have physical and chemical properties resembling calcium ?
(CBSE All India 2011)
Answer:
Only those elements are placed in the same group in which the gaps of atomic numbers are : 8, 8, 18, 18, 32. If we look at the atomic numbers of the elements that are listed, it becomes clear that the elements with atomic numbers 12, 20 (Ca), 38 fit into this pattern. They are placed in the same group and have also similar physical and chemical properties.
Question 10.
Compare and contrast the arrangement of elements in Mendeleevs periodic table and the modern periodic table.
Answer:
The main points of distinction between Mendeleevs periodic table and Modern periodic table are as follows :
| Mendeleev’s Periodic Table | Modern Periodic Table |
| 1. It regards atomic masses of the elements as the basis of classification. | It regards atomic number of the elements as the basis of classification. |
| 2. No separate positions or slots have been allotted to the isotopes of an element since they have different atomic masses. | There is no need for the separate slots for the isotopes since they have the same atomic numbers. |
| 3. No justification is made for placing hydrogen in group IA along with alkali metals. | Justification has been made for placing hydrogen along with alkali metals in group 1 since both hydrogen and alkali metals have one valence electron. |
| 4. Except for the elements in group VIII, the remaining groups have been divided with sub-groups A and B. | There are no sub-groups and all groups one independent in nature. |
| 5. Position of certain elements based on their atomic masses are misfits. For example, the element cobalt (atomic mass = 58-9) has been its placed ahead of nickel (atomic mass = 58-7) | Modern periodic table is free from such anomalies. The element cobalt is placed before nickel since its atomic number (27) is less than that of nickel (28). |
| 6. Electronic configurations and properties of the elements can not be predicted from their positions to the table. | Both electronic configuration and certain properties of the elements can be predicted from their positions in the periodic table. |
| 7. It is not very systematic and is difficult to remember. | It is quite systematic and is easy to remember. |
CBSE Class 10 Science Notes Chapter 5 Periodic Classification of Elements
“The periodic table is a tabular method of displaying the elements in such a way, that the elements having similar properties occur in the same vertical column or group”.
Earlier attempts of the classification of elements: Dobereiner’s Triads, Newland’s law of octaves.
Dobereiner’s Triads: This classification is based on the atomic mass. According to this, when elements are arranged in order of increasing atomic masses, groups of three elements, having similar properties are obtained. The atomic mass of middle element of the triad being nearly equal to the average of the atomic masses of the other two elements.
For Example Li (6.9), Na (23), K (39).
Limitation: It fails to arrange all the known elements in the form of triads, even having similar properties.
Newland’s Law of Octaves: According to this ‘when elements are placed in order of increasing atomic masses, the physical and chemical properties of every 8th element are a repetition of the properties of the first element.’
Form of Newland’s octaves is given in the following table:

Limitations
- Law of octaves was applicable only upto calcium (only for lighter elements).
- Newland adjusted two elements in the same slot (e.g. Co and Ni), having different properties. For example; Co and Ni with Fluorine, Chlorine, Bromine and Iodine.
- According to Newland, only 56 elements existed in nature and no more elements would be discovered in future.
Present attempts for the classification of elements: Mendeleev’s Periodic Table, the Modern Periodic Table.
Mendeleev’s Periodic Table: Mendeleev’s periodic table is based on the physical and chemical properties of elements and their atomic masses.
Mendeleev’s Periodic Law: According to this “The physical and chemical properties of the elements are the periodic function of their atomic masses.”
Periodicity of Properties: The repetition of properties of elements after certain regular intervals is known as Periodicity of Properties.
Merits of Mendeleev’s Periodic Table
- Mendeleev’s left vacant places in his table which provided an idea for the discovery of new elements. Example: Eka-boron, Eka-aluminium and Eka-silicon.
- Mendeleev’s periodic table was predicted properties of several undiscovered elements on the basis of their position in Mendeleev’s periodic table.
- It is useful in correcting the doubtful atomic masses of some elements.
- Noble gases could accommodate in the Mendeleev’s periodic table without disturbing the periodic table after discovery.
Limitations of Mendeleev’s Periodic Table
(a) No fixed position for hydrogen: No correct position of the hydrogen atom was in Mendeleev’s periodic table.
Example: Position of hydrogen with alkali metals and halogens (17th group).
(b) No place for isotopes: Position of isotopes were not decided.
Example: Cl-35 and Cl-37.
(c) No regular trend in atomic mass: Position of some elements with lower atomic masses before with higher atomic mass.
Example: Ni-58.7 before Co-58.9.
Mendeleev’s original periodic table is reproduced in the table below

The Modern Periodic Table: In 1913, Henry Moseley showed that the atomic number of an element is a more fundamental property than its atomic mass.
Modern Period Law: The physical and chemical properties of elements are the periodic function of their atomic number.
Modern periodic table is based on atomic number of elements.
Atomic number (Z) is equal to the number of protons present in the nucleus of an atom of an element.
Modern periodic table contains 18 vertical column known as group and seven horizontal rows known as periods.
On moving from left to right in a period, the number of valence electrons increases from 1 to 8 in the elements present.
On moving from left to right in a period, number of shell remains same.
All the elements of a group of the periodic table have the same number of valence electrons.
Trends in Modern Periodic Table: Valency, Atomic size, metallic and non-metallic characters, and Electronegativity.
(i) Valency: The valency of an element is determined by the number of valence electrons present in the outermost shell of its atom (i.e. the combining capacity of an element is known as its valency).
In Period: On moving from left to right in a period, the valency first increases from 1 to 4 and then decreases to zero (0).

In Groups: On moving from top to bottom in a group, the valency remains same because the number of valence electrons remains the same.
Example: Valency of first group elements = 1 Valency of second group elements = 2.
(ii) Atomic size: Atomic size refers to radius of an atom. It is a distance between the centre of the nucleus and the outermost shell of an isolated atom.
In Period : On moving from left to right in a period, atomic size decreases because nuclear charge increases.
Example: Size of second period elements: Li > Be > B > C > N > O > F
Point to know: The atomic size of noble gases in corresponding period is largest
due to presence of fully filled electronic configuration (i.e. complete octet).
In Group: Atomic size increases down the group because new shells are being
added in spite of the increase in nuclear charge.
Example ; Atomic size of first group element : Li < Na < K < Rb < Cs < Fr
Atomic size of 17th group elements : F < Cl < Br < I
(iii) Metallic character: It is the tendency of an atom to lose electrons. In Period: Along the period from left to right, metallic characters decreases because a tendency to lose electron decreases due to the increase in nuclear charge. Example: Metallic character of second period elements: Li > Be > B > C >> N > O > F
In Group: Metallic character, when moving from top to bottom increases because the atomic size and tendency to lose electrons increases.
Example: First group element : Li < Na < K < Rb < Cs

17th group elements: F < Cl < Br < I
(iv) Non-metallic character: It is tendency of an atom to gain electrons.
In Period: Along the period from left to right, non-metallic character increases because tendency to gain electrons increases due to increase in nucleus charge. Example ; Non-metallic character of 2nd period elements : Li < Be < B < C < N < O < F In Group: On moving from top to bottom in a group, non-metallic character decreases because atomic size increases and tendency to gain electrons decreases. Ex. Non-metallic character of 17th period element: F > Cl > Br > I
(v) Chemical Reactivity
In metals: Chemical reactivity of metals increases down the group because tendency to lose electrons increases. Example ; Li < Na < K < Rb < Cs (1st group) In non-metals: Chemical reactivity of non-metals decreases down the group because tendency to gain electrons decreases. Example: F > Cl > Br > I (17th group)
(vi) Electronegativity: It is tendency of an element to attract the shared pair of electrons towards it in a covalently bonded molecule. It increases with increase of nuclear charge or decrease in atomic size.
Along the period electronegativity increases. Example ;Li < Be < B < C < N < O < F. Down the group electronegativity decreases. Example ; Li > Na > K > Rb > Cs
F > Cl > Br > I
(vii) Nature of Oxides: Metal oxides are basic in nature. Ex. Na2O, MgO etc.
Non-metal oxides are acidic in nature. Ex. Cl2O7, SO3, P2O5,
In the case of metal reactivity, it increases down the group because of the tendency to lose electrons increases.
In the case of non-metal reactivity, decreases down the group because of the tendency to gain electrons decreases.
Group: The vertical columns in Mendeleev’s, as well as in Modern Periodic Table, are called groups.
Period: The horizontal rows in the Modern Periodic Table and Mendeleev’s Periodic Table are called periods.
There are 18 groups and 7 (seven) periods in the Modern Periodic Table.
Atomic size: The atomic size may be visualised as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
The trend of atomic size (radius) in moving down a group: Ongoing down in a group of the Periodic Table, the atomic size increases because a new shell of electrons is added to the atoms at every step. There is an increase in distance between the outermost shell electrons and the nucleus of the atom.
The trend of atomic size (radius) in moving from left to right in a period: On moving from left to right along a period, the size of atoms decreases because on moving from left to right, the atomic number of elements increases which means that the number of protons and electrons in the atoms increases. Due to the large positive charge on the nucleus, the electrons are pulled in more closely to the nucleus and the size of the atom decreases.
Characteristics of triads of J.W. Dobereiner.
- Elements of a triad show similar chemical properties.
- These elements of a triad show specific trends in their physical properties.
- The atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
Example: Atomic mass of Na is 23 in the triad Li, Na and K. This atomic mass is the average of the atomic masses of Li and K which have atomic masses 7 and 39 respectively.
Triads as formed by Dobereiner.
1st Triad
Li – Lithium
Na – Sodium
K – Potassium
2nd Triad
Ca – Calcium
Sr – Strontium
Ba – Barium
3rd Triad
Cl – Chlorine
Br – Bromine
I – Iodine
Mendeleev’s Periodic Law: It states that “the properties of elements are the periodic functions of their atomic masses.” It means the properties of the elements depend on their atomic masses and the elements are given a position in the periodic table on the basis of their increasing atomic masses.
Merits of Mendeleev’s Periodic Table
(i) Mendeleev left a number of gaps in his table to accommodate the new elements which would be discovered later on. So Mendeleev boldly predicted the existence of some more elements. He even predicted the properties of some of these elements and named them as Eka-boron, Eka-aluminium and Eka-silicon respectively. Later on the elements were discovered, for example, gallium replaced Eka-aluminium and it showed properties similar to that of aluminium.
(ii) He gave the proper position to the noble gases which were discovered later on, without disturbing the existing order of elements. He placed them in a new group.
Limitations of Mendeleev’s classification:
- The position of isotopes could not be explained because isotopes have the same chemical properties but different atomic masses. If the elements are arranged according to atomic masses, the isotopes should be placed in different groups of the Periodic Table.
- The atomic masses do not increase in a regular manner in going from one element to the next.
- He could not assign a correct position to hydrogen in his table because hydrogen has some properties similar to alkali metals and some properties similar to halogens.
Modem Periodic Law: This law was proposed by Henry Moseley, a scientist in 1913. According to this Law, “Properties of elements are the periodic function of their atomic number.” It means that the properties of elements depend on their atomic number and the elements are given positions in the periodic table on the basis of their increasing atomic number. As atomic number determines the distribution of electrons in the orbits, and electrons of the outermost orbit determine the properties of an element.
Groups and periods in the Modem (long form) Periodic Table: There are 18 groups (vertical columns) and 7 periods (horizontal lines) in the Modern (or long form) Periodic Table. The number of the period is equal to the number of shells in the atoms of the elements belonging to that period.
Trends in Mendeleev’s Periodic Table
- The properties of elements are periodic functions of their atomic mass.
- It has 8 groups.
- No place could be assigned to isotopes of an element.
- There were three gaps left by Mendeleev in his Periodic Table.
- No fixed position was given to hydrogen in this Periodic Table.
- No distinction was made between metals and non-metals.
- Transition elements are placed together in Group VIII.
- Inert gases were not known at the time of Mendeleev.
Trends in Modem Periodic Table
(i) Valency: Elements belonging to the same group have the same number of valence electrons and thus the same valency. Valency in a particular period from left to right first increases as positive valency and then decreases as negative valency.
Example: In elements of 2nd period:
Li has 1+ valency, then Be2+, B3+, C4+ covalency, N3- valency, then O2- and F(-) valency.
(ii) The atomic size or atomic radius increases: as we move down in a group and it decreases as we move from left to right in a period. Atomic size increases down a group due to the increase in the number of shells. Atomic size decreases along a period due to an increase in the nuclear charge which tends to pull the electrons closer to the nucleus and reduces the size of the atom.
(iii) Metallic and Non-Metallic properties: In the modern periodic table metals are on the left side and non-metals on the right side of the table. A zig-zag line of metalloids separates metals from non-metals.
- Metallic characters decrease from left to right in a period and increase while going down in a group.
- Non-metallic characters increase from left to right in a period due to increase in the electronegativity and these characters decrease from top to bottom in a group due to the decrease in the electronegativity of atoms while going down in a group.
1. Need for classification of elements:
Increase in the discovery of different elements made it difficult to organise all that was known about the elements. To study a large number of elements with ease, various attempts were made. The attempts resulted in the classification of elements into metals and non-metals.
2. Dobereiner’s triads:
Johann Wolfgang Dobereiner, a German chemist, classified the known elements in groups of three elements on the basis of similarities in their properties. These groups were called triads.
(i) Characteristics of Triads:
- Properties of elements in each triad were similar.
- Atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
(ii) Examples of Triads:

(iii) Limitations: Dobereiner could identify only three triads. He was not able to prepare triads of all the known elements.
3. Newlands’ Law of Octaves:
John Newlands’, an English scientist, arranged the known elements in the order of increasing atomic masses and called it the ‘Law of Octaves’. It is known as ‘Newlands’ Law of Octaves’.
(i) Characteristics of Newlands’ Law of Octaves:
- It contained the elements from hydrogen to thorium.
- Properties of every eighth element were similar to that of the first element.
(ii) Table showing Newlands’ Octaves:

(iii) Limitations of Newlands’ law of Octaves:
- The law was applicable to elements up to calcium (Ca).
- It contained only 56 elements.
- In order to fit elements into the table, Newlands’ adjusted two elements like cobalt and nickel in die the same slot and also put some unlike elements under the same note.
4. Mendeleev’s Periodic Table: Dmitri Ivanovich – 5 ’ Mendeleev, a Russian demist, was the most important contributor to the early development of a periodic table of elements wherein the elements were arranged on the basis of their atomic mass and chemical properties.
- Characteristics of Mendeleev’s Periodic Table:
- Mendeleev arranged all the 63 known elements in increasing order of their atomic masses.
- The table contained vertical columns called ‘groups’ and horizontal rows called ‘periods’.
- The elements with similar physical and chemical properties came under the same groups.
- Mendeleev’s Periodic Law: The properties of elements are the periodic function of their atomic masses.
- Achievements of Mendeleev’s Periodic Table:
- Through this table, it was very easy to study the physical and chemical properties of various elements.
- Mendeleev adjusted few elements with a slightly greater atomic mass before the elements with slightly lower atomic mass, so that elements with similar properties could be grouped together. For example, aluminium appeared before silicon, cobalt appeared before nickel.
- Mendeleev left some gaps in his periodic table.
He predicted the existence of some elements that had not been discovered at that time. His predictions were quite true as elements like scandium, gallium and germanium were discovered later. - The gases like helium, neon and argon, which were discovered later, were placed in a new group without disturbing the existing order.
- Limitations:
- No fixed positions were given to hydrogen in the Mendeleev’s periodic table.
- Positions of Isotopes of all elements was not certain according to Mendeleev’s periodic table.
- Atomic masses did not increase in a regular manner in going from one element to the next.
5. Modem Periodic Table: Henry Moseley, gave a new ! property of elements, ‘atomic number’ and this was I adopted as the basis of Modem Periodic Table.
(i) Modem Periodic Law: Properties of elements are a periodic function of i their atomic number.
(ii) The position of elements in Modem Periodic Table:
- The modem periodic table consists of 18 groups and 7 periods.
- Elements present in any one group have the same number of valence electrons. Also, the number of shells increases as we go down the group.
- Elements present in any one period, contain the same number of shells. Also, with increase in atomic number by one unit on moving from left to right, the valence shell electrons increases by one unit.
- Each period marks a new electronic shell getting filled.
(iii) Table showing Electronic Configuration of First 20 Elements:

Trends in the Modern Periodic Table:
- Valency: Valency of an element is determined by the number of valence electrons present in the outermost shell of its atom.
- Valency of elements in a particular group is same.
- Valency of elements in a period first increases from one to four and then decreases to zero.
- Atomic Size: Atomic size refers to the radius of an atom.
- In a period, atomic size and radii decreases from left to right.
- In a group, atomic size and radii increases from top to bottom.
Metallic and Non-metallic Properties:
-
- The tendency to lose electrons from the outermost shell of an atom, is called metallic character of an element.
- The tendency to gain electrons from the outermost shell of an atom, is called non-metallic character of an element.

NCERT Exemplar Class 10 Science Chapter 5 Periodic Classification of Elements
Short Answer Questions
Question.1 “Hydrogen occupies a unique position in the Modern Periodic Table”. Justify the statement.
Answer. Hydrogen resembles with both group 1 (Alkali metals) as well as group 17 (Halogens), therefore, it occupies a unique position in the Modern Periodic Table.
Question.2 Write the formula of chlorides of Eka-silicon and Eka-aluminium, the elements predicted by Mendeleev.
Answer.
Germanium is Eka-silicon. The formula isGeCl4.
Gallium is Eka-aluminium. The formula is GaCl3.
Question.3 Three elements A, B and C have 3, 4 and 2 electrons respectively in their outermost shell. Give the group number to which they belong in the Modern Periodic Table. Also, give their valencies
Answer.

Question.4 If an element X is placed in group 14, what will be the formula and the nature of bonding of its chloride?
Answer.‘X’ belongs to group 14, it has four valence electrons and valency is equal to 4.

The nature of bond is covalent.
Question.5 Compare the radii of two species X and Y. Give reasons for your answer.
(a) X has 12 protons and 12 electrons
(b) Y has 12 protons and 10 electrons
Answer. ‘X’ has 12 protons and 12 electrons. It has bigger atomic size as compared to
‘Y’ which has 12 protons and 10 electrons because effective nuclear charge is less in X than Y. Secondly, number of shells are more in ‘X’ than ‘Y’.
Therefore,
(a) ‘X’ has bigger atomic size.
(b) Y has smaller size as compared to ‘X’.
Question.6 Arrange the following elements in increasing order of their atomic radii,
(a) Li, Be, F, N
(b) Cl, At, Br, I
Answer. (a) F < N < Be < Li is increasing order of atomic size because atomic size decreases along a period from left to right.
(b) Cl < Br < I < At is increasing order of atomic size because atomic size increases down the group due to increase in number of shells.
Question.7 Identify and name the metals out of the following elements whose electronic configurations are given below.
(a) 2, 8, 2 (b) 2, 8. 1
(c) 2.8. 7 (d) 2. 1
Answer. (a) Magnesium (b) Sodium (c) Chlorine (d) Lithium
Metals are (a) Magnesium, (b) Sodium and (d) Lithium as they have 1 to 3 valence electrons. Whereas, (c) chlorine is a non-metal.
Question.8 Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
Answer.

Question.9 Arrange the following elements in the increasing order of their metallic character: Mg, Ca, K, Ge, Ga.
Answer. Ge < Ga < Mg < Ca < K is increasing order of metallic character. It is because ‘K’ can lose electron more easily due to larger size whereas, ‘Ge’ is smallest and cannot lose electrons.
Long Answer Questions
Question.10. An element is placed in 2nd Group and 3rd Period of the Periodic Table, burns in presence of oxygen to form a basic oxide,
(a) Identify the element.
(b) Write the electronic configuration.
(c) Write the balanced equation when it burns in the presence of air.
(d) Write a balanced equation when this oxide is dissolved in water.
(e) Draw the electron dot structure for the formation of this oxide.
Answer.

Question.11. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the periodic table are elements X and Y placed?
(b) Classify X and Y as metal(s), non- metal(s) or metalloid(s).
(c) What will be the nature of oxide of element Y? Identify the nature of bonding in the compound formed.
(d) Draw the electron dot structure of the divalent halide.
Answer.

(a) ‘X’ is in group 17 and 3rd period, ‘Y’ is in group 2 and 4th period.
(b) ‘X’ is non-metal and ‘Y’ is a metal.
(c) ‘Y’ forms basic oxide. It has ionic bonding in the compound formed.
(d) :X“Y”X: is the electron dot diagram.
Question.12. Atomic number of a few elements are given below.
10, 20, 7, 14
(a) Identify the elements. .
(b) Identify the Group number of these elements in the Periodic Table.
(c) Identify the Periods of these elements in the Periodic Table.
(d) What would be the electronic configuration for each of these elements?
(e) Determine the valency of these elements.
Answer.

Question.13 (i) Electropositive nature of the elements(s) increases down the group and decreases across the period.
(ii) Electronegativity of the element decreases down the group and increases across the period.
(iii) Atomic size increases down the group and decreases across a period (left to right).
(iv) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
(a) Name the most electropositive element among them.
(b) Name the most electronegative element.
(c) Name the element with smallest atomic size.
(d) Name the element which is a metalloid.
(e) Name the element which shows maximum valency.
Answer.
(a) Lithium (3)
(b) Fluorine (9)
(c) Fluorine (9)
(d) Boron (5)
(e) Carbon (6). Its valency is 4.
Question.14 An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
(a) Identify the element X.
(b) Write the electronic configuration of X.
(c) Write the balanced chemical equa-tion for the thermal decomposition of ferrous sulphate crystals.
(d) What would be the nature (acidic/ basic) of oxides formed?
(e) Locate the position of the element in the Modern Periodic Table.
Answer.

Question.15 An element X of group 15 exists as diatomic molecule and combines with hydrogen at 773 K in presence of the catalyst to form a compound, ammonia which has a characteristic pungent smell.
(a) Identify the element X. How many valence electrons does it have?
(b) Draw the electron dot structure of the diatomic molecule of X. What type of bond is formed in it?
(c) Draw the electron dot structure for ammonia and what type of bond is formed in it? .
Answer.

NCERT Exemplar Solutions for Class 10 Science Chapter 5 Periodic Classification of Elements
Question 1.
Upto which element, the Law of Octaves was found to be applicable ?
(a) Oxygen
(b) Calcium
(c) Cobalt
(d) Potassium.
Answer:
(b). Law was found to be applicable upto the element calcium.
Question 2.
According to Mendeleev’s Periodic Law, the elements were arranged in the periodic table in the order of
(a) increasing atomic number
(b) decreasing atomic number
(c) increasing atomic masses
(d) decreasing atomic masses.
Answer:
(c).
Question 3.
In Mendeleev’s Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the periodic table later ?
(a) Germanium
(b) Chlorine
(c) Oxygen
(d) Silicon.
Answer:
(a). The element was called Eka-silicon.
Question 4.
Which of the following statement (s) about the Modern Periodic Table are incorrect ?
(i) The elements in the Modern Periodic Table are arranged on the basis of their decreasing atomic numbers
(ii) The elements in the Modern Periodic Table are arranged on the basis of their increasing atomic masses.
(iii) Isotopes are placed in adjoining group (s) in the Periodic Table
(iv) The elements in the Modern Periodic Table are arranged on the basis of their increasing atomic numbers
(a) (i) only
(b) (i), (ii) and (iii)
(c) (i), (ii) and (iv)
(d) (iv) only.
Answer:
(b). Statements (i), (ii) and (iii) are all in correct.
Question 5.
Which of the following statements about the Modern Periodic Table is correct ?
(a) It has 18 horizontal rows known as Periods
(b) It has 7 vertical columns known as Periods
(c) It has 18 vertical columns known as Groups
(d) It has 7 horizontal rows known as Groups.
Answer:
(c).
Question 6.
Which of the given elements A, B, C, D and E with atomic numbers 2, 3, 7, 10 and 30 respectively belong to the same period ?
(a) A, B, C
(b) B, C, D
(c) A, D, E
(d) B, D, E.
Answer:
(b). The elements B (Z = 3), C (Z = 7) and D(Z = 10) belong to the same period. It is second period.
Question 7.
The elements A, B, C, D and E have atomic numbers 9, 11, 17, 12 and 13 respectively. Which pair of elements belong to the same group ?
(a) A and B
(b) B and D
(c) A and C
(d) D and E.
Answer:
(c). Elements A (Z = 9) and C (Z = 17) belong to the same group. It is a halogen family (group 17).
Question 8.
Where would you locate the element with electronic configuration 2, 8 in the Modern Periodic Table ?
(a) Group 8
(b) Group 2
(c) Group 18
(d) Group 10.
Answer:
(c). It is a noble gas element Neon (Ne) present in group 18.
Question 9.
An element which is an essential constituent of all organic compounds belongs to
(a) group 1
(b) group 14
(c) group 15
(d) group 16.
Answer:
(b). The element is carbon. It belongs to group 14.
Question 10.
Which of the following is the outermost shell for elements of period 2 ?
(a) K shell
(b) L shell
(c) M shell
(d) N shell
Answer:
(b). In second (2) period, the electrons are filled in second shell also known as L-shell.
Question 11.
Which one of the following elements exhibits maximum number of valence electrons ?
(a) Na
(b) Al
(c) Si
(d) P
Answer:
(d). The element phosphorus (P) has five electrons (2, 5) in the valence shell.
Question 12.
Which of the following gives the correct increasing order of the atomic radii of O, F and N ?
(a) O, F, N
(b) N, F, O
(c) O, N, F
(d) F, O, N.
Answer:
(d). It is the correct order. These elements are present in second period.
Question 13.
Which among the following elements has the largest atomic radius ?
(a) Na
(b) Mg
(c) K
(d) Ca.
Answer:
(c). The element potassium (K) present in group 1 has the largest atomic radius.
Question 14.
Which of the following elements would lose an electron easily ?
(a) Mg
(b) Na
(c) K
(d) Ca.
Answer:
(c). The element potassium (K) with maximum size would lose electron easily.
Question 15.
Which of the following elements does not lose an electron easily ?
(a) Na
(b) F
(c) Mg
(d) Al.
Answer:
(b). The element fluorine (F) with smallest size does not lose electron easily.
Question 16.
Which of the following are the characteristics of isotopes of an element ?
(i) Isotopes of an element have same atomic mass.
(ii) Isotopes of an element have same atomic number.
(iii) Isotopes of an element show same physical properties.
(iv) Isotopes of an element show same chemical properties.
(a) (i), (iii) and (iv)
(b) (ii), (iii) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv).
Answer:
(d).
Question 17.
Arrange the following elements in the order of their decreasing metallic character Na, Si, Cl, Mg, Al
(a) Cl > Si > Al > Mg > Na
(b) Na > Mg > Al > Si > Cl
(c) Na > Al > Mg > Cl > Si
(d) Al > Na > Si > Ca > Mg.
Answer:
(b). The metallic character of the elements decreases along a period. These elements are present in third period.
Question 18.
Arrange the following elements in the order of their increasing non-metallic character Li, O, C, Be, F
(a) F < O < C < Be < Li
(b) Li < Be < C < O < F
(c) F < O < C < Be < Li
(d) F < O < Be < C < Li.
Answer:
(b). The non-metallic character of the elements increases along a period. These elements are present in second period in the order Li, Be, C, O, F.
Question 19.
What type of oxide would Eka-aluminium form ?
(a) EO3
(b) E3O2
(c) E2O3
(d) EO.
Answer:
(c). Is the correct answer.
Question 20.
Three elements B, Si and Ge are
(a) metals
(b) non-metals
(c) metalloids
(d) metal, non-metal and metalloid respectively.
Answer:
(c). is the correct answer. These are also called semi-metals and possess the characteristics of both metals and non-metals.
Question 21.
Which of the following elements will form an acidic oxide ?
(a) An element with atomic number 7
(b) An element with atomic number 3
(c) An element with atomic number 12
(d) An element with atomic number 19.
Answer:
(a). The element with atomic number (Z) = 7 is nitrogen. It forms acidic oxides such as N2O3, N2O5 etc.
All other elements are metals and they form basic oxides.
Question 22.
The element with atomic number 14 is hard and forms acidic oxide and a covalent halide. To which of the following categories does the element belong ?
(a) Metal
(b) Metalloid
(c) Non-metal
(d) Left-hand side element.
Answer:
(b). The element with atomic number (Z) = 14 is silicon (Si). It is a metalloid.
Question 23.
Which one of the following depicts the correct representation of atomic radius (r) of an atom ?
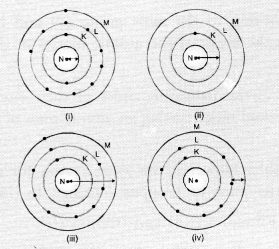
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv).
Answer:
(b). The atom (ii) has only one shell (K-shell). No electrons are present in the other shells. Therefore, the arrow represents correct atomic radius.
The arrow in atom (iii) also represents the correct atomic radius of the element.
Question 24.
Which one of the following does not increase while moving down the group of the periodic table ?
(a) Atomic radius
(b) Metallic character
(c) Valence
(d) Number of shells in an element.
Answer:
(c). In a group, the valence or valency does not change since all the elements present have same valence shell configuration.
Question 25.
On moving from left to right in a period in the periodic table, the size of the atom
(a) increases
(b) decreases
(c) does not change appreciably
(d) first decreases and then increases
Answer:
(b). Atomic size decreases along a period. However, the noble gas atom is an exception. It has very large size.
Question 26.
Which of the following set of elements is written in order of their increasing metallic character ?
(a) Be Mg Ca
(b) Na Li K
(c) Mg Al Si
(d) C O N
Answer:
(a). These elements belong to group 2. These are written in increasing order of their size. Since the metallic character increases down the group, the order is the correct.
NCERT Exemplar Solutions for Class 10 Science Chapter 5 Short Answer Questions
Question 27.
The three elements A, B and C with similar properties have atomic masses X, Y and Z respectively. The mass of Y is approximately equal to the average mass of X and Z. What is such an arrangement of elements called as ? Give one example of such a set of elements.
Answer:
The arrangement is known as Dobereiner’s triad. For example, Calcium (Ca), Strontium (Sr) and Barium (Ba).
Question 28.
Elements have been arranged in the following sequence on the basis of their increasing atomic masses.
F, Na, Mg, Al, Si, P, S, Cl, Ar, K
(a) Pick two sets of elements which have similar properties.
(b) The given sequence represents which law of classification of elements ?
Answer:
(a) The elements that have similar properties belong to the same group. From the list of elements available, elements which belong to same group are : .
Na, K (Alkali metals) ; F, Cl (Halogens)
(b) The sequence is according to Newland’s law of octaves.
Question 29.
Can the following groups of elements be classified as Dobereiner’s triad ?
(a) Na, Si, Cl
(b) Be, Mg, Ca
Answer:
Atomic mass of Be = 9; Na = 23; Mg = 24; Si = 28; Cl = 35; Ca = 40 Explain by giving reason.
(a) No, these elements cannot be classified as triads because these do not have same properties. However, the atomic mass of Si (28) is almost the mean of the atomic masses of elements Na (23) and Cl (35).
Note : The Dobereiner’s triad is meaningful only if the elements present in the triad have almost identical properties.
(b) Yes, these elements can be classified as triads. These belong to the same group (2) and have almost identical properties. The atomic mass of Mg (24) is almost the mean of the atomic masses of the elements Be (9) and Ca(40) i.e., 9 + 40 = 49/2 = 24.5.
Question 30.
In Mendeleev’s Periodic Table, the elements were arranged in the increasing order of their atomic masses. However, cobalt with atomic mass of 58.93 amu was placed before nickel having an atomic mass of 58.71 amu. Give reason for the same.
Answer:
This is regarded as a defect in the Mendeleev’s periodic table. However, the main reason for making the arrangement was that the elements with similar characteristics must be grouped together. In this case,
• Cobalt (Co) should be in the company of the elements Rhodium (Rh) and Iridium (Ir).
• Nickel (Ni) should be in the company of the elements Palladium (Pd) and Platinum (Pt).
Question 31.
“Hydrogen occupies a unique position in Modern Periodic Table”. Justify the statement.
Answer:
The position of the element hydrogen is still not clear even in the Modern Periodic Table.
• In electronic configuration, it resembles alkali metals of group 1. All of them have only one electron in the valence shell. Actually, hydrogen has only one shell (K-shell) which has one electron. However, it is not a metal whereas alkali-metals are metallic in nature.
• In characteristics, it resembles halogens of group 17. For example, like halogens hydrogen is a non-metal and diatomic as well.
It has been therefore, decided to assign hydrogen a unique position in the Modern or Long Form. Periodic Table. It is placed at the top in group 1 of alkali metals. However, it is not a member of that group.*
Question 32.
Write the formulae of chlorides of Eka-silicon and Eka-aluminium, the ^elements predicted by Mendeleev.
Answer:
Eka-aluminium represents gallium (Ga) with valency three and Eka-silicon is for germanium (Ge) with valency four. The formulae of their respective chlorides are GaCl3 and GeCl4.
Question 33.
Three elements A, B and C have 3, 4 and 2 electrons respectively in their outermost shell. Give the group number to which they belong in the Modern Periodic Table. Also, give their valencies.
Answer:
The electrons present in the outermost shell are also known as valence electrons. The desired information is given :

Question 34.
If an element X is placed in group 14, what will be the formula and the nature of bonding of its chloride ?
Answer:
The element X present in group 14 has four valence electrons in its atom. It can complete its octet by sharing four valence electrons with the electrons of other atoms. Therefore, it will form covalent bonds with the four atoms of chlorine. The formula of the chloride of the element X is.
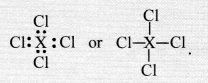
Question 35.
Compare the radii of two species X and Y. Give reasons for your answer.
(a) X has 12 protons and 12 electrons
(b) Y has 12 protons and 10 electrons
Answer:
The available information makes it clear that :
(a) Species X with equal number of protons (12) and electrons (12) is an atom.
(b) Species Y with two electrons less (10) than the number of protons (12), is a divalent cation y2+ of species X.
Therefore, the radius of species X is more as compared to that of y2+.
Note : The radius of cation is always less than that of atom while that of anion is more.
Question 36.
Arrange the following elements in increasing order of their atomic radii.
Answer:
(a) All the elements belong to the same period (second). The atomic size or radius decreases along a period. Therefore, increasing order of atomic radii is : F < N < Be < Li
(b) The listed elements are present in group 17 (halogen family). The atomic size or radius increases down
a group. Therefore, the correct increasing order of atomic radii is : Cl < Br < I < At.
Question 37.
Identify and name the metals out of the following elements whose electronic configurations are given below :
(a) 2, 8, 2
(b) 2, 8, 1
(c) 2, 8, 7
(d) 2, 1.
Answer:
(a) Electronic configuration : 2, 8, 2 (Mg is a metal)
(b) Electronic configuration : 2, 8, 1 (Na is a metal)
(c) Electronic configuration : 2, 8, 7 (Cl is a non-metal)
(d) Electronic configuration : 2, 1 (Li is a metal)
Question 38.
Write the formula of the compound formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. ^What is the nature of the bond formed ? (CBSE 2013)
Answer:
Electronic configuration of element A (Z = 19) is 2, 8, 8, 1
Electronic configuration of element B(Z = 17) is 2, 8, 7.
The element A has one valence electron while the element B has seven electrons in its valence shell. One electron gets transferred from the atom of element A to the atom of element B. As a result of electron transfer, an ionic bond is formed.
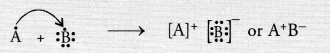
Question 39.
Arrange the following elements in the increasing order of their metallic character. ,
Mg, Ca, K, Ge, Ga
Answer:
In general, the metallic character increases down the group and decreases along a period. From the relative positions of the elements in the periodic table, the increasing order of metallic character is :
Ge < Ga < Mg < Ca < K.
Question 40.
Identify the elements with the following property and arrange them in increasing order of their reactivity
(a) An metal which is soft and reactive
(b) The metal which is an important constituent of limestone
(c) The metal which exists in liquid state at room temperature
Answer:
(a) The metal may be either Na or K.
(b) The metal is Ca.
(c) The metal is Hg.
Question 41.
The increasing order of reactivity of the metals is :
Hg < Ca < Na < K.
Properties of the elements are given below. Where would you locate the following elements in the periodic table ?
(a) A soft metal stored under kerosene
(b) An element with variable (more than one) valency stored under water
(c) An element which is tetravalent and forms the basis of organic chemistry
(d) An element which is an inert gas with atomic number 2
(e) An element whose thin oxide layer is used to make other elements corrosion resistant by the process of “anodising.”
Answer:
(a) Sodium (Group 1 and Period 3) or Potassium (Group 1 and Period 4)
(b) Phosphorus (Group 15 and Period 3). It shows variable valencies 3 and 5 and is stored under water.
(c) Carbon (Group 14 and Period 2)
(d) Helium (Group 18 and Period 1)
(e) Aluminium (Group 13 and Period 3)
NCERT Exemplar Solutions for Class 10 Science Chapter 5 Long Answer Questions
Question 42.
An element placed in 2nd group and 3rd Period of the Periodic Table, burns in the presence of oxygen to form a basic oxide.
(a) Identify the element
(b) Write the electronic configuration
(c) Write the balanced equation when it burns in the presence of air
(d) Write a balanced equation when this oxide is dissolved in water
(e) Draw the electron dot structure for the formation of this oxide
Answer:
(a) The element is magnesium (Mg).
(b) The electronic configuration is 2, 8, 2.
(c) Magnesium burns in oxygen (air) to form magnesium oxide which is of basic nature.
2Mg(s) + O2(g) ————-> 2MgO(s)
(d) Magnesium hydroxide is formed.
MgO(s) + H2O(aq) ————> Mg(OH)2(s)
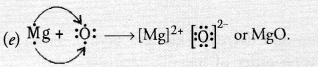
Question 43.
An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the Periodic Table are elements X and Y placed ?
(b) Classify X and Y as metal(s), non-metal(s) or metalloid(s).
(c) What will be the nature of oxide of element Y ? Identify the nature of bonding in the compound formed
(d) Draw the electron dot structure of the divalent halide.
Answer:
Element X (Z = 17) is chlorine while element Y (Z = 20) is calcium.
(a) Chlorine (Cl) is a member of group 17 and period 3. Calcium (Ca) is present in group 2 and period 4.
(b) Chlorine is a typical non-metal while calcium is a metal.
(c) The oxide of calcium is calcium oxide (CaO). It is a basic oxide.
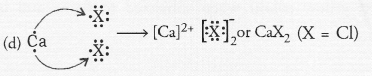
Question 44.
Atomic number of a few elements are : 10, 20, 7, 14
(a) Identify the elements
(b) Identify the Group number of these elements in the Periodic Table
(c) Identify the Periods of these elements in the Periodic Table
(d) What would be the electronic configuration for each of these elements ?
(e) Determine the valency of these elements.
Answer:
(a) The elements are : Neon (Z = 10), Calcium (Z = 20), Nitrogen (Z = 7) and Silicon (Z = 14)
(b) Group numbers : Neon (18), Calcium (2), Nitrogen (15), Silicon (14).
(c) Periods : Neon (2), Calcium (4), Fluorine (2), Silicon (3).
(d) Electronic Configuration : Neon (2, 8) ; Calcium (2, 8, 8, 2) Nitrogen (2, 5) ; Silicon (2, 8, 4)
(e) Valency : Neon (zero) ; Calcium (2) ; Nitrogen (3) ; Silicon (4)
Question 45.
(a) In this ladder symbols of elements are jumbled up. Rearrange these symbols of elements in the increasing order of their atomic number in the Periodic Table.
(b) Arrange them in the order of their group also.
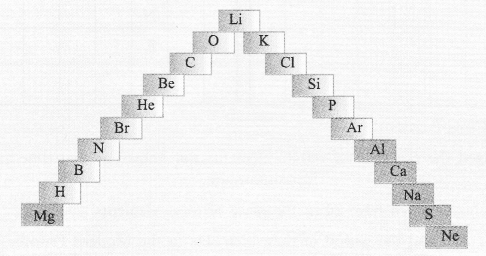
Answer:
(a) H, He, Li, Be, B, C, N, O, Ne, Na, Mg, Al, Si, P, S, Cl, Ar, K, Ca, Br.
(b) Group 1 — H, Li, Na, K
Group 2 — Be, Mg, Ca
Group 13 — B, A1
Group 14 — C, Si
Group 15 — N, P
Group 16 — O, S
Group 17 — Cl, Br
Group 18 — He, Ne, Ar
Question 46.
Complete the following cross word puzzle
Across :
(1) An element with atomic number 12.
(3) Metal used in making cans and member of Group 14.
(4) A lustrous non-metal which has 7 electrons in its outermost shell.
Down :
(2) Highly reactive and soft metal which imparts yellow colour when subjected to flame and is kept in kerosene.
(5) The first element of second period
(6) An element which is used in making fluorescent bulbs and is second member of group 18 in the Modern Periodic Table.
(7) A radioactive element which is the last member of the halogen family.
(8) Metal which is an important constituent of steel and forms rust when exposed to moist air.
(9) The first metalloid in Modern Periodic Table whose fibres are used in making bullet proof vests.
| 1 | 7 | 2 | |||||||||
| 3 | 8 | 9 | 5 | ||||||||
| 4 | 6 | ||||||||||
(Cross-Puzzle)
Answer:
| 1M | 7A | G | N | E | 2s | I | U | M | |||
| S | O | ||||||||||
| 3T | 8I | N | D | 9B | 5L | ||||||
| A | R | 4I | o | D | I | 6n | E | ||||
| T | O | U | R | T | E | ||||||
| I | N | M | O | H | O | ||||||
| N | N | I | N | ||||||||
| E | U | ||||||||||
| M |
(Puzzle Solved)
Question 47.
Mendeleev predicted the existence of certain elements not known at that time and named two of them as Eka-silicon and Eka-aluminium.
(a) Name the elements which have taken the place of these elements.
(b) Mention the group and the period of these elements in the Modern Periodic Table.
(c) Classify these elements as metals, non-metals or metalloids.
(d) How many valence electrons are present in each one of them ?
Answer:
(a) Germanium (Ge) for Eka-silicon and gallium (Ga) for Eka-aluminium
(b) Germanium (Group 14 and Period 4)
Gallium (Group 13 and Period 4)
(c) Germanium (Metalloid) ; Gallium (Metal)
(d) Germanium (Z = 32), Four valence electrons (2, 8, 18, 4)
Gallium (Z = 31) ; Three valence electrons (2, 8, 18, 3).
Question 48.
(a) Electropositive nature of the element(s) increases down the group and decreases across the period
(b) Electronegativity of the element decreases down the group and increases across the period
(c) Atomic size increases down the group and decreases across a period (left to right)
(d) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
(a) Name the most electropositive element among them.
(b) Name the most electronegative element.
(c) Name the element with smallest atomic size.
(d) Name the element which is a metalloid.
(e) Name the element which shows maximum valency.
Answer:
(a) Lithium (Z = 3) is the most electropositive element,
(b) Fluorine (Z = 9) is the most electronegative element.
(c) Fluorine (Z = 9) has the smallest atomic size.
(d) Boron (Z = 5) is a metalloid.
(e) Carbon (Z = 6) shows the maximum valency (4). However, the element nitrogen (Z = 7) can show valency 5 in some compounds (e.g., N2O5).
Question 49.
An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
(a) Identify the element X.
(b) Write the electronic configuration of X.
(c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals.
(d) What would be the nature (acidic/basic) of oxides formed ?
(e) Locate the position of the element in the Modern Periodic Table.
Answer:
(a) The available information suggests that the element X is sulphur.
(b) Electronic configuration of S(Z = 16) 2, 8, 6
![]()
(d) Fe2O3 (basic oxide), SO2(acidic oxide), SO3(acidic oxide)
(e) Sulphur is a member of group 16 and period 3.
Question 50.
An element X of group 15 exists as diatomic molecule and combines with hydrogen at 773 K in presence of the catalyst to form a compound, ammonia which has a characteristic pungent smell.
(a) Identify the element X. How many valence electrons does it have ?
(b) Draw the electron dot structure of the diatomic molecule of X. What type of bond is formed in it ?
(c) Draw the electron dot structure for ammonia. What type of bonds is formed in it ?
Answer:
(a) The available information suggests that the element X is nitrogen (N) and exists in diatomic form as N,
Electronic configuration of N(Z = 7) ; 2, 5. It has five valence electrons.
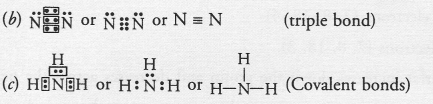
Question 51.
Which group of elements could be placed in Mendeleev’s Periodic Table without disturbing the original order ? Give reason.
Answer:
Group of noble gases (called zero group) could be placed in the Mendeleev’s Periodic Table with out disturbing the original order.
Reasons : Elements present in a group have same valency in the Mendeleev’s Periodic Table. Based on their electronic configuration, the members of noble gas family have zero valency. That is why they are called inert gases. They could be easily placed in the Mendeleev’s Periodic Table as a separate group without disturbing the arrangement of other elements.
Question 52.
Give an account of the process adopted by Mendeleev for the classification of elements. How did he arrive at “Periodic Law” ?
Answer:
- The basis of classification of elements adopted was atomic masses of the elements. The elements were arranged in order of increasing atomic masses.
- Elements with similar properties were kept in a particular group in order of increasing atomic masses.
- Elements placed in a particular group (or sub group) were having same valency.
Note : Please note that when Mendeleev arranged the elements in the Periodic Table only 63 elements were known. Even noble gas or inert gas elements were adjusted at a later stage. Moreover, many groups were left in the table.
