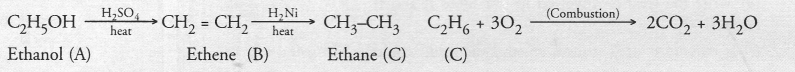NCERT Solutions for Class 10 Science Chapter 4 Carbon and its Compounds
NCERT Solutions for Class 10 Science Chapter 4 Intext Questions
Page Number: 61
Question 1
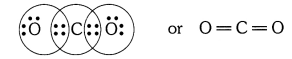
What would be the electron dot structure of carbon dioxide which has the formula CO2 ?
Answer:
Question 2
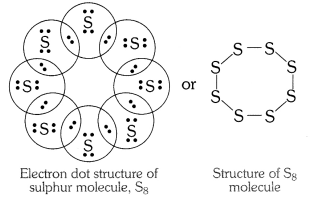
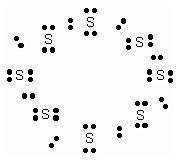
What would be electron dot structure of sulphur which is made up of eight atoms of sulphur.
Answer:
Page Number: 68 – 69
Question 1
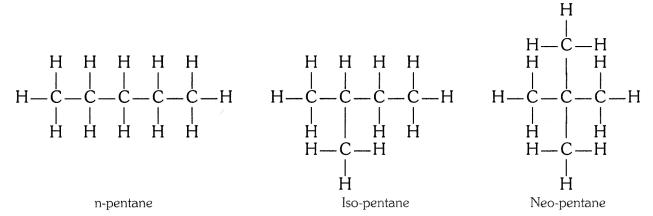
How many structural isomers can you draw for pentane ?
Answer:
Three, these are n-pentane, iso-pentane and neo-pentane.
Question 2
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us ?
Answer:
(i) Tetravalency
(ii) Catenation.
Question 3
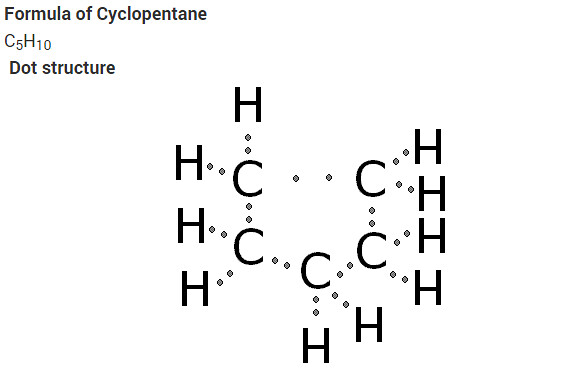
What will be the formula and electron dot structure of cyclopentane ?
Answer:
The molecular formula of cyclopentane is C5 H10 .
The electron dot structure of cyclopentane is given on the next page.
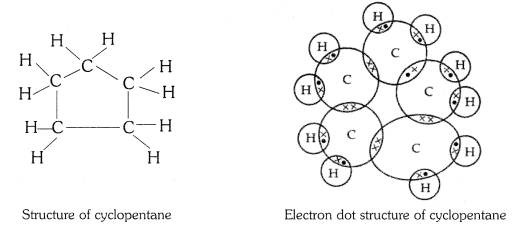
Question 4
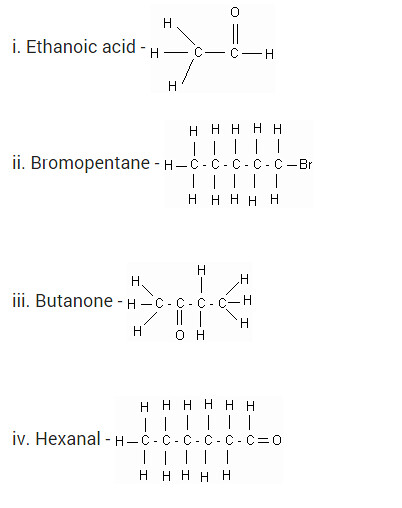
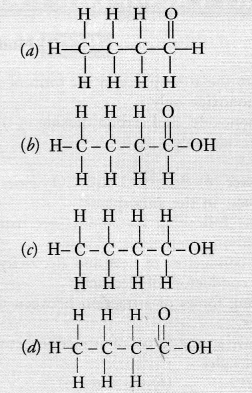
Draw the structures for the following compounds :
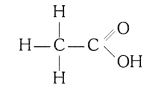
(i) Ethanoic acid
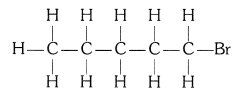
(ii) Bromopentane
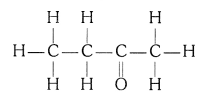
(iii) Butanone
(iv) Hexanal
Answer:
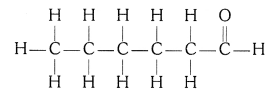
(i) Ethanoic acid (CH3COOH)
(ii) Bromopentane (C5H11Br)
(iii) Butanone (CH3 — CH2 — COCH3)
(iv) Hexanal (C5H11CHO)
Structural isomers for bromopentane: There are three structural isomers for bromopentane depending on the position of Br at carbon 1, 2, 3.
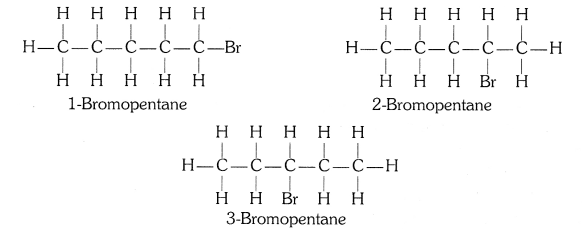
Positions 4 and 5 are same as 1, 2.
Question 5
How would you name the following compounds ?
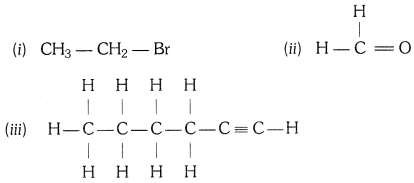
Answer:
(i) Bromoethane
(ii) Methanal
(iii) 1 – Hexyne
Page Number: 71
Question 1
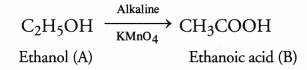
Why is the conversion of ethanol to ethanoic acid an oxidation reaction ?
Answer:
Conversion of ethanol into ethanoic acid is an oxidation reaction because addition of oxygen to a substance is called oxidation. Here, oxygen is added to ethanol by oxidising agent like alkaline potassium permanganate or acidified potassium dichromate and it is converted into acid.

Question 2
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used ?
Answer:
A mixture of ethyne and air is not used for welding because burning of ethyne in air produces a sooty flame due to incomplete combustion, which is not enough to melt metals for welding.
Page Number: 74
Question 1
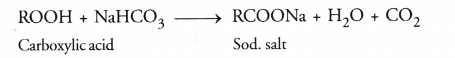
How would you distinguish experimentally between an alcohol and a carboxylic acid ?
Answer:
Differences between alcohol and carboxylic acid
| Test | Alcohol | Carboxylic acid |
| (i) Litmus test | No change in colour. | Blue litmus solution turns red. |
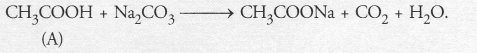
| (ii) Sodium hydrogen carbonate test | C2H5OH + NaHCO3 → No reaction No brisk effervescence. | CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 Brisk effervescence due to evolution of CO2. |
| (iii) Alkaline potassium permanganate | On heating, pink colour disappears. | Does not happen so. |
Question 2
What are oxidising agents ?
Answer:
Oxidising agents are the substances which give oxygen to another substances or which remove hydrogen from a substance.
For example, acidic K2Cr2O7 is an oxidising agent, that converts (oxidises) ethanol into ethanoic acid.
Page Number: 76
Question 1
Would you be able to check if water is hard by using a detergent ?
Answer:
No, because detergents can lather well even in hard water. They do not form insoluble calcium or magnesium salts (scum). On reacting with the calcium ions and magnesium ions present in the hard water.
Question 2
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes ?
Answer:
It is necessary to agitate to get clean clothes because the soap micelles which entrap oily or greasy particles on the surface of dirty cloth have to be removed from its surface. When the cloth wetted in soap solution is agitated or beaten, the micelles containing oily or greasy dirt get removed from the surface of dirty cloth and go into water and the dirty cloth gets cleaned.
NCERT Solutions for Class 10 Science Chapter 4 Textbook Chapter End Questions
Question 1
Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer:
(b) 7 covalent bonds.
Question 2
Butanone is a four-carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c) Ketone.
Question 3
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer:
(b) The fuel is not burning completely.
Question 4
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
Covalent bond is formed by sharing of electrons so that the combining atoms complete their outermost shell.
In CH3Cl : C = 6, H = 1 and Cl = 17 And their electronic configuration is C – 2,4, H – 1 and Cl – 2, 8, 7
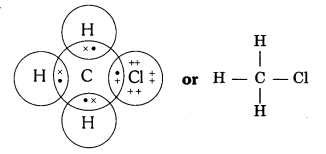
Three hydrogen atoms complete their shells by sharing three electrons (one electron each) of carbon atom.
Chlorine completes its outer shell by sharing its one out of seven electrons with one electron of carbon atom.
Thus carbon atom shares all its four electrons with three hydrogen atoms and one of chlorine atom and completes its outermost shell and single covalent bonds are formed in CH3Cl.
Question 5
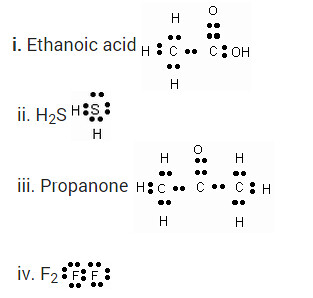
Draw the electron dot structures for
(a) ethanoic acid
(b) propanone
(c) H2S
(d) F2.
Answer:
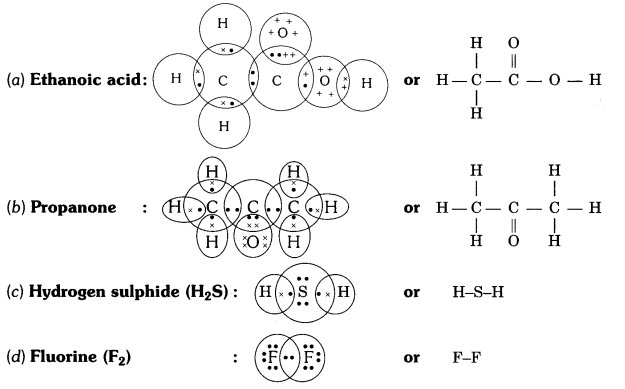
Question 6
What is a homologous series ? Explain with an example.
Answer:
Homologous series : A homologous series is a group of organic compounds having
similar structures and similar chemical properties in which the successive compounds differ by -CH2 group.
Characteristics of homologous series :
(i) All members of a homologous series can be represented by the same general formula. For example, the general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes number of carbon and hydrogen atoms in one molecule of alkane.
(ii) Any two adjacent homologues differ by one carbon atom and two hydrogen atoms in their molecular formulae.
(iii) The difference in the molecular masses of any two adjacent homologues is 14u.
(iv) All the compounds of a homologous series show similar chemical properties.
(v) The members of a homologous series show a gradual change in their physical properties with increase in molecular mass.
For example, general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes number of carbon atoms in one molecule of alkane. Following are the first five members of the homologous series of alkanes (general formula CnH2n+2).
| Value of n | Molecular formula | Name of compound |
| 1 | CH4 | Methane |
| 2 | C2H6 | Ethane |
| 3 | C3H8 | Propane |
| 4 | C4H10 | Butane |
| 5 | C5H12 | Pentane |
Question 7
How can ethanol and ethanoic acid he differentiated on the basis of their physical and chemical properties ?
Answer:
Difference on the basis of physical properties
| Property | Ethanol | Ethanoic acid |
| (i) State | Liquid | Liquid |
| (ii) Odour | Sweet smell | Pungent vinegar-like smell |
| (iii) Melting point | 156 K | 290 K |
| (iv) Boiling point | 351 K | 391 K |
Difference on the basis of chemical properties
| Test | Ethanol | Ethanoic acid |
| (i) Litmus test | No change in the colour of litmus solution. | Blue litmus solution turns red. |
| (ii) Sodium hydrogen carbonate test | C2H5OH + NaHCO3 → No reaction No brisk effervescence. | CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 Brisk effervescence due to evolution of CO2. |
| (iii) Alkaline potassium permanganate | On heating, pink colour disappears. | Does not happen so. |
Question 8
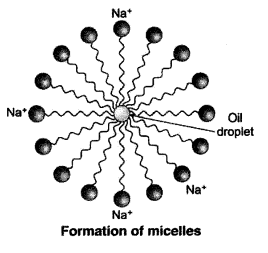
Why does micelle formation take place when soap is added to water ? Will a micell be formed in other solvents such as ethanol also ?
Answer:
Micelle formation takes place when soap is added to water because the hydrocarbon chains of soap molecules are hydrophobic (water repelling) which are insoluble in water, but the ionic ends of soap molecules are hydrophilic (water attracting) and hence soluble in water.
Such micelle formation will not be possible in other solvents like ethanol in which sodium salt of fatty acids do not dissolve.
Question 9
Why are carbon and its compounds used as fuels for most applications ?
Answer:
Carbon and its compounds give a large amount of heat per unit weight and are therefore, used as fuels for most applications.
Question 10
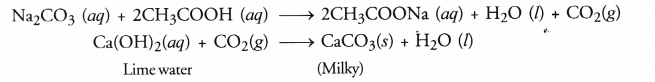
Explain the formation of scum when hard water is treated with soap.
Answer:
Hard water contains salts of calcium and magnesium. Calcium and magnesium on reacting with soap form insoluble precipitate called scum. The scum formation lessens the cleansing property of soaps in hard water.
Question 11
What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
Red litmus will turn blue because soap is alkaline in nature. Blue litmus remains blue in soap solution.
Question 12
What is hydrogenation ? What is its industrial application ?
Answer:
The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydro-carbon is called hydrogenation. The process of hydrogenation takes place in the presence of nickel (Ni) or palladium (Pd) metals as catalyst.
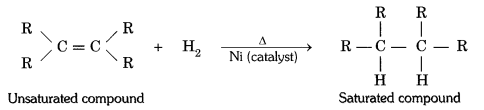
Application : The process of hydrogenation has an important industrial application. It is used to prepare vegetable ghee (or vanaspati ghee) from vegetable oils.
Question 13
Which of the following hydrocarbons undergo addition reactions :
C2H6, C3H8, C3H6, C2H2 and CH4
Answer:
Addition reactions take place only in unsaturated hydrocarbons. So addition reaction take place only in C3H6 and C2H2.
Question 14
Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer:
Butter is a saturated carbon compound while cooking oil is an unsaturated carbon compound. An unsaturated compound decolourises bromine water, while a saturated compound cannot decolourise it. So we can distinguish chemically between a cooking oil and butter by the bromine water. Add bromine water to a little of cooking oil and butter taken in separate test-tubes.
- Cooking oil decolourises bromine water showing that it is an unsaturated compound.
- Butter does not decolourise bromine water showing that it is a saturated compound.
Question 15
Explain the mechanism of the cleaning action of soaps.
OR
Explain the cleansing action of soaps. [CBSE 2015 (Delhi)]
Answer:
When a dirty cloth is put in water containing dissolved soap, then the hydrocarbon end of the soap molecules in micelle attach to the oil or grease particles present on the surface of dirty cloth. In this way the soap micelle entraps the oily or greasy particles by using its hydrocarbon ends. The ionic ends of the soap molecules in the micelles, however, remain attached to water. When the dirty cloth is agitated in soap solution, the oily and greasy particles present on its surface and entrapped by soap micelles get dispersed in water due to which the soap water becomes dirty but the cloth gets cleaned. The cloth is cleaned thoroughly by rinsing in clean water a number of times.
NCERT Solutions for Class 10 Science Chapter 4 Carbon and its Compounds
Carbon compounds: Covalent bonding in carbon compounds, Versatile nature of carbon, Homologous series, Nomenclature of carbon compounds containing functional groups, (halogens, alcohol, ketones, aldehydes, alkanes, and alkynes), difference between saturated hydrocarbons and unsaturated hydrocarbons. Chemical properties of carbon compounds (combustion, oxidation, addition and substitution reaction). Ethanol (only properties and uses), Ethanoic acid (only properties and uses), soaps and detergents.
Question 1
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Solution:
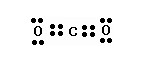
Question 2
What would be the electron dot structure of a molecule of sulphur, which is made up of eight atoms of sulphur? (Hint – The eight atoms of sulphur are joined together in the form of a ring.)
Solution:
Question 3
How many structural isomers can you draw for pentane?
Solution:
We can draw 3 structural isomers for pentane.
Question 4
What are the two properties of carbon that lead to the huge number of carbon compounds we see around us?
Solution:
Due to its large valency, carbon atoms can form covalent bonds with a number of carbon atoms as well as with a large number of other atoms such as hydrogen, oxygen, nitrogen, sulphur, chlorine and many more atoms. This leads to the formation of a large number of organic compounds.
Question 5
What will be the formula and electron dot structure of Cyclopentane?
Solution:
Question 6
Draw the structures for the following compounds.
i. Ethanoic acid
ii. Bromopentane
iii. Butanone
iv. Hexanal
Solution:
Question 7
How would you name the following compounds?
Solution:
i. Ethyl bromide
ii. Formaldehyde
iii. Hexyne
Question 8
Why is the conversion of ethanol to Ethanoic acid an oxidation reaction?
Solution:
The conversion of ethanol into ethanoic acid is called an oxidation reaction because oxygen is added to it during this conversion.

Question 9
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Solution:
When a mixture of oxygen and ethyne is burnt, it burns completely producing a blue flame. This blue flame is extremely hot which produced a very high temperature which is used for welding metals. But the mixture of ethyne and air is not used for welding purposes because burning of ethyne in air produces a sooty flame, which is not enough to melt metals for welding.
Question 10
What are oxidizing agents?
Solution:
Oxidizing agents are the substances that gain electrons in an redox reaction and whose oxidation number is reduced.
Question 11
Explain the nature of the covalent bond using the bond formation of CH3Cl.
Solution:
CH3Cl(methyl chloride) is made up of one carbon atom, three hydrogen atoms and one chlorine atom. Carbon atom has 4 valence electrons, each hydrogen atom has one valence electron, and a chlorine atom has 7 valence electrons. Carbon atom shares its four valence electrons with three hydrogen atoms and 1 chlorine atom to form methyl chloride as follows:

From the above reaction, in the dot structure of methyl chloride (CH3Cl) there are four pairs of shared electrons between carbon and other atoms. Each pair of shared electrons constitutes one single covalent bond. So, methyl chloride has four single covalent bonds.
Question 12
Draw the electron dot structures for-
Solution:
Question 13
What is a homologous series? Explain with an example.
Solution:
Homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and shows a gradation in physical properties as a result of increase in molecular size and mass. For example, methane has a lower boiling point than ethane since it has more intermolecular forces with neighbouring molecules. This is because of the increase in the number of atoms making up the molecule.
Question 14
How can ethanol and Ethanoic acid be differentiated on the basis of their physical and chemical properties?
Solution:
(i) Ethanol has a pleasant smell whereas ethanoic acid has the smell of vinegar.
(ii) Ethanol has a burning taste whereas ethanoic acid has a sour taste.
(iii) Ethanol has no action on litmus paper whereas ethanoic acid turns blue litmus paper red.
(iv) Ethanol has no reaction with sodium hydrogencarbonate but Ethanoic acid gives brisk effervescence with sodium hydrogencarbonate.
Question 15
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Solution:
Micelle formation takes place when soap is added to water. This is because when soap is added to water in which dirty clothes are soaked, the two parts of the soap molecule dissolves in two different mediums. The organic tail dissolves in the dirt, grime or grease and the ionic head dissolves in water. When the clothes are rinsed or agitated, the dirt gets pulled out of the clothes in the water by the soap molecule. In this way the soap does its cleaning work on dirty and grimy clothes or hands.
The soap molecules actually form a closed structure because of mutual repulsion of the positively charged heads. This structure is called a micelle.
Question 16
Why are carbon and its compounds used as fuels for most applications?
Solution:
Carbon and its compounds are used as fuels for most of the applications because they burn in air releasing a lot of heat energy.
Question 17
Explain the formation of scum when hard water is treated with soap.
Solution:
The precipitate form of scum is formed when soap is used for washing clothes. With hard water, a large amount of soap is wasted in reacting with the calcium and magnesium ions of hard water to form an insoluble precipitate. The precipitate form formed by the action of hard water on soap, sticks to the clothes being washed and interferes with the cleaning ability of the additional soap. This makes the cleaning of clothes difficult.
Question 18
What change will you observe if you test soap with litmus paper (red and blue)?
Solution:
Soap is the salt of a strong base (NaOH) and a weak acid (carboxylic acid), so a solution of soap in water is basic in nature. Being basic, a soap solution turns red litmus paper blue.
Question 19
What is hydrogenation? What is its industrial application?
Solution:
It is a class of chemical reactions in which the net result is addition of hydrogen (H2) to unsaturated organic compounds such as alkenes, alkynes, etc. Hydrogenation is widely applied to the processing of vegetable oils and fats. Complete hydrogenation converts unsaturated fatty acids to saturated ones.
Question 20
C2H5, C3H8, C3H6, C2H2 and CH4
Solution:
Alkenes and alkynes (unsaturated hydrocarbons) undergo addition reactions. From the above hydrocarbons C2H2 is an alkyne, whereas C3H6 is an alkene. So, C3H6 and C2H2 will undergo addition reactions.
Question 21
Give a test that can be used to differentiate chemically between butter and cooking oil.
Solution:
Bromine water test can be used to differentiate chemically between butter and cooking oil. Add bromine water to a little of cooking oil and butter taken in separate test tubes. <font
a. Decolourising of bromine water by cooking oil (unsaturated compound)
b. Butter (saturated compound) does not decolourise bromine water
Question 22
Explain the mechanism of the cleaning action of soaps.
Solution:
We all know that soap is used to remove dirt and and grime from substances. Generally dirt and grime get stuck because they have an oily component, which is difficult to remove, by plain brushing or washing by water. A soap molecule has two parts, a head and a tail i.e. the long chain organic part and the functional group –COO– Na+.
A soap molecule has a tadpole like structure shown below.
The organic part is water insoluble but is soluble in organic solvents or in oil or grease. The ionic part is soluble in water, as water is a polar solvent. When soap is added to water in which dirty clothes are soaked, the two parts of the soap molecule dissolve in two different mediums. The organic tail dissolves in the dirt, grime or grease and the ionic head dissolves in water. When the clothes are rinsed or agitated, the dirt gets pulled out of the clothes, by the soap molecule. In this way soap does its cleaning work on dirty and grimy clothes or hands.
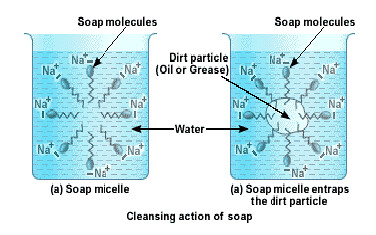
The soap molecules actually form a closed structure because of mutual repulsion of the positively charged heads. This structure is called a micelle. The micelle pulls out the dirt and grime more efficiently.
Question 23
Would you be able to check if water is hard by using a detergent?
Solution:
We would not be able to check whether a sample of water is hard by using a detergent, this is because a detergent forms lather easily even with hard water.
Question 24
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat ii with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Solution:
It is necessary to shake to get clean clothes because the soap micelles, which entrap oily or greasy particles on the surface of dirty clothes, have to be removed from their surface. When the clothes which are wet by soap solution are beaten, the micelles containing oil or greasy dirt particles get removed from the surface of dirty clothes and go into water and the dirty cloth gets cleaned.
Multiple Choice Questions (MCQs) [1 Mark each]
Question 1.
Buckminster fullerene is an allotropic form of [NCERT Exemplar]
(a) phosphorus
(b) sulphur
(c) carbon
(d) tin
Answer:
(c) Buckminster fullerene is an allotrope of carbon containing clusters of 60 carbon atoms joined together to form spherical molecules. Its formula isC60 (C-sixty). It is a dark solid at room temperature and as compared to another allotropic form of carbon (diamond and graphite), it is neither very hard nor soft.
Question 2.
The hetero atoms present in
CH3 – CH2 – O – CH2 – CH2Cl are [NCERT Exemplar]
(i) oxygen
(ii) carbon
(iii) hydrogen
(iv) chlorine
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer:
(d) Atoms other than C and H, if present in organic compound, are called heteroatoms.
Question 3.
In which of the following .compounds -OH is the functional group? [NCERT Exemplar]
(a) Butanone
(b) Butanol
(c) Butanoic
(d) Butanal
Answer:
(b) Butanol, CH3—CH2—CH2—CH2—OH
The general formula of alcohols is CnH2n+1— OH.
For butanol, n = 4. So, formula is
C4H9—OH or CH3—CH2—CH2—CH2—OH
Question 4.
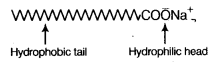
The soap molecule has a [NCERT Exemplar]
(a) hydrophilic head and a hydrophobic tail
(b) hydrophobic head and a hydrophilic tail
(c) hydrophobic head and a hydrophobic tail
(d) hydrophilic head and a hydrophilic tail
Answer:
(a) A soap molecule is made up of two parts- a long hydrocarbon part and a short ionic part —COONa+ group. The long hydrocarbon chain is hydrophobic (water repelling) and ionic portion is hydrophilic (water attracting).
Question 5.
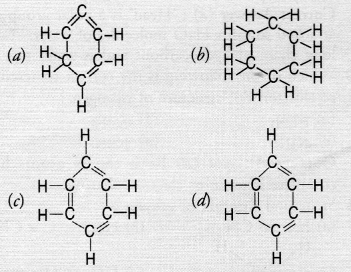
Structural formula of benzene is [NCERT Exemplar]
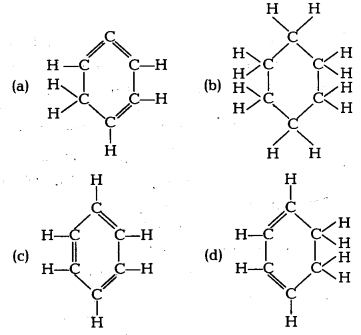
Answer:
(c) Benzene molecule contains alternate single and . double bonds. Its formula is C6H6. In structure (b) formula is C6H12. In structure (a) double bond is not at alternate position. In (d) formula is C6H8.
Question 6.
Which of the following is not a straight chain hydrocarbon? [NCERT Exemplar]
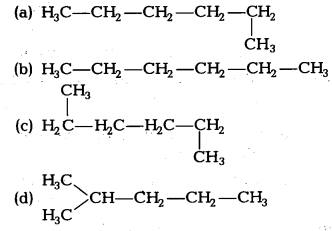
Answer:
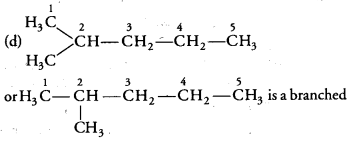
chain hydrocarbon not straight chain hydrocarbon. Rest three are straight chain hydrocarbons.
Question 7.
Which among the following are unsaturated hydrocarbons? [NCERT Exemplar]
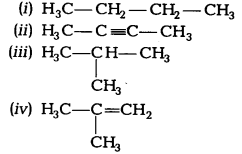
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer:
(c) Unsaturated hydrocarbons have double or triple bond in the structure. Both (ii) and (iv) structures have triple and double carbon-carbon bonds respectively.
Question 8.
Chlorine reacts with saturated hydrocarbons at room temperature in the [NCERT Exemplar]
(a) absence of sunlight
(b) presence of sunlight
(c) presence of water
(d) presence of hydrochloric acid
Answer:
(b) Chlorine reacts with saturated hydrocarbon at room temperature in the presence of sunlight.
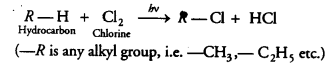
Question 9.
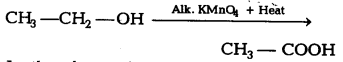
In the above given reaction, alk.KMnO4 acts as [NCERT Exemplar]
(a) reducing agent
(b) oxidising agent
(c) catalyst agent
(d) dehydrating
Answer:
(b) KMnO4 acts as oxidising agent, because it removes hydrogen from CH3CH2OH and adds one oxygen to it.
Question 10.
Butanone is a four carbon compound with functional group [NCERT Exemplar]
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c) In butanone, the functional group is
![]()
Question 11.
Identify the unsaturated compounds from the following [NCERT Exemplar]
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (ii) and (iii)
Answer:
(d) Propene, CH3CH=CH2 (ii) and propyne, CH3— C = CH (iii) both have double and triple bonds, respectively, hence are unsaturated compounds.
Question 12.
Which of the following does not belong to the same homologous series? [NCERT Exemplar]
(a) CH4
(b) C2H6
(c) C3H8
(d) C4H8
Answer:
(d) Because succesive members of a homologous series differ by —CH2 unit.
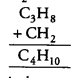
Thus, C4H10 is the next member of this series. So, homologous series of alkanes is:
methane (CH4), ethane (C2H6), propane (C3H8) and butane (C4H10).
So, C4H8 does not belong to the homologous series.
Question 13.
Ethane with molecular formula C2H6 has [NCERT Exemplar]
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer:
(b) Structure formula of ethane (C2H6) is
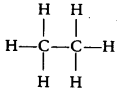
It is clear that it has 7 covalent bonds.
Question 14.
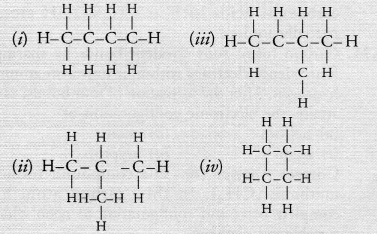
Which of the following are correct structural isomers of butane? [NCERT Exemplar]
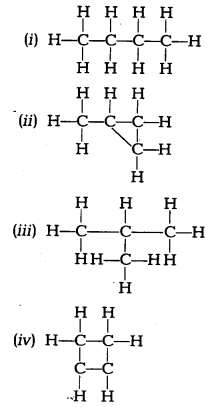
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:
(a) Structure (i) is n-butane and structure (iii) is iso-butane. Since, molecular formula is same, only structures are different. So, (i) and (iii) are isomers while structures (ii) and (iv) have molecular formulaC4H8.
Question 15.
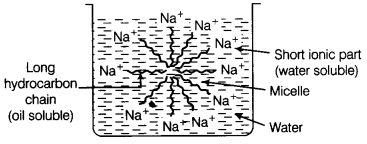
In the soap micelles, [NCERT Exemplar]
(a) the ionic end of soap is on the surface of the cluster while the carbon chain is in the interior of the cluster
(b) ionic end of soap is in the interior of the cluster and the carbon chain is out of the cluster
(c) Both ionic end and carbon chain are in the interior of the cluster
(d) Both ionic end and carbon chain are on the exterior of the cluster
Answer:
(a) A ‘spherical aggregate of soap molecules’ in the soap solution in water is called a ‘micelle’. In a soap micelle, the soap molecules are arranged readily with hydrocarbon ends directed towards the centre and ionic ends directed outwards.
Question 16.
Vinegar is a solution of [NCERT Exemplar]
(a) 50% – 60% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 50% – 60% acetic acid in water
Answer:
(c) A 5%-8% solution of acetic acid in water is called vinegar.
Question 17.
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of [NCERT Exemplar]
(a) addition reaction
(b) substitution reaction
(c) displacement reaction
(d) oxidation reaction
Answer:
(a) Oils are unsaturated compounds containing double bonds. Addition reactions are characteristic property of unsaturated hydrocarbons. The given reaction is an example of addition reaction.
Question 18.
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of [NCERT Exemplar]
(a) helium
(b) neon
(c) argon
(d) krypton
Answer:
(b) Electronic configuration of carbon (C) = 2, 4 when it forms four covalent bonds by sharing its four valence electrons with hydrogen, it forms CH4 molecule like this
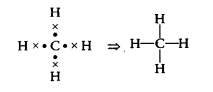
Now, electronic configuration of C in CH
4 = 2, 8.
Atomic number of Ne is 10. Its electronic K L configuration is 2,8. Therefore, after the formation of four bonds, carbon attains the electronic configuration of neon.
Question 19.
Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised.
(ii) carboxylic acids are completely ionised.
(iii) mineral acids are partially ionised.
(iv) carboxylic acids are partially ionised.
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:
(a) Mineral acids are strong acids which ionise almost completely and carboxylic acids are weak acids which ionise only pardally.
Question 20.
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that [NCERT Exemplar]
(a) food is not cooked completely
(b) the fuel is not burning completely
(c) fuel is wet
(d) fuel is burning completely
Answer:
(b) The unburnt particles of the fuel present in smoke blacken the vessel from outside.
Question 21.
The reaction in which a reagent (partially or completely) replaces atom or group of atoms from saturated compounds or A are called B reaction.
Here, A and B respectively refers to
(a) unsaturated compounds, addition
(b) unsaturated compounds, substitution
(c) benzene, substitution
(d) alkene, addition
Answer:
(c) Substitution reaction is usually given by saturated compounds and benzene. Unsaturated compounds usually give addition reactions.
Question 22.
The table gives information about some esters and the fragrance they produce.
| Ester | Fragrance |
| Ethyl methanoate | Rum |
| Methyl butanoate | Apple |
| Ethyl butanoate | Pineapple |
| Propyl ethanoate | Pear |
Which structure do the ester compounds in the table have in common?
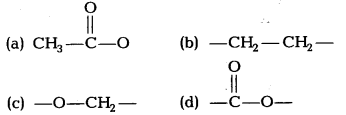
Answer:
(d) All esters have the common structure of carboxylic group represented by the suffix date.
Carbon and its Compounds Class 10 Important Questions and Answers Science Chapter 4
Very Short Answer Questions
Question 1.
What are the essential constituents of all organic compounds ?
Answer:
Carbon and hydrogen are the essential constituents of all organic compounds. However, carbon tetrachloride (CCl4) is an exception.
Question 2.
What is the valency of carbon in its compounds ?
Answer:
Carbon is tetravalent in its compounds.
Question 3.
Why are organic compounds present in such a large number ?
Answer:
This is due to the self linking property of carbon known as catenation.
Question 4.
Which is common in all the members of a family ?
Answer:
They have the common functional group.
Question 5.
A family of organic compounds has the functional group ‘al’. What is its name ?
Answer:
The family is of aldehydes also called alkanals.
Question 6.
Out of ketonic and aldehydic groups, which is the terminal functional group ?
Answer:
Aldehydic group

Question 7.
Why is candle flame generally yellow ?
Answer:
Candle flame is generally yellow due to the presence of unburnt carbon particles. When light falls on these particles, they scatter yellow colour. This shows that the combustion of hydrocarbons present in wax or candle is not complete.
Question 8.
The formula of a hydrocarbon is CnH2n. Name the family to which it belongs and also predict its nature.
Answer:
The hydrocarbon belongs to alkene family. It is unsaturated in nature.
Question 9.
An unknown compound has the smell of vinegar. Identify it.
Answer:
The compound is ethanoic acid also called acetic acid.
Question 10.
What do we get when ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid ?
Answer:
Ethyl ethanoate (CH3COOC2H5) is formed by esterification reaction. It has fruity smell.
Question 11.
Name the second member of alkyne family. Give its structure.
Answer:
The second member of alkyne family is propyne. Its structural formula is H3C—C = CH.
Question 12.
Vapours of a hydrocarbon were passed through bromine dissolved in carbon tetrachloride. The yellow colour of bromine got discharged ? Predict the nature of the hydrocarbon.
Answer:
The hydrocarbon is unsaturated. It is either an alkene or alkyne.
Question 13.
Give a test to identify the presence of ethanoic acid.
Answer:
Dip a strip of blue litmus paper in the solution of ethanoic acid. Its colour will change to red.
Question 14.
Out of butter and ground nut oil, which is unsaturated in nature ?
Answer:
Ground nut oil is unsaturated in nature.
Question 15.
What is the role of soap in cleansing of clothes ?
Answer:
Soap helps in forming a stable emulsion between oil drops carrying dirt particles and water. The emulsion is also known as micelle.
Question 16.
Which organic compound is added to make ethanol unfit for drinking purposes ? What is the name of the mixture formed ?
Answer:
Methanol which is highly poisonous is added in small amount to ethanol in order to make it unfit for drinking purposes. The mixture is called methylated spirit or denatured alcohol.
Question 17.
Can you check hard water by using a detergent ?
Answer:
No, it is not possible because detergents give lather with both soft and hard waters.
Question 18.
When do you get yellow soot in the burner flame ?
Answer:
Yellow soot is obtained when the holes of the burner are not clean. The combustion is incomplete. The yellow – soot or yellow flame is because of unburnt carbon particles.
Question 19.
Write IUPAC and common names of CH3COCH3, C2H5COOH.
Answer:
CH3COCH3 : Propanone, Acetone
C2H5COOH : Propanoic acid, Propionic acid.
Question 20.
Which of the following belong to the same homologous series ?
C3H8, C4H8, C4H6, C3H6.
Answer:
C3H6 and C4H8 belong to the same homologous series which is alkenes with general formula CnH2n.
Question 21.
Which has a triple bond; C2H2, C3H4 and ?
Answer:
C3H4 has triple bond with the formula CH3C ≡ CH
Question 22.
The molecular formula of butane is C4H10. What is the formula of butene ?
Answer:
The formula of butene is C4H8.
Question 23.
A compound with molecular forumla C2H6O is used as a fuel. Identify the compound.
Answer:
The compound is ethanol with formula C2H5OH.
Question 24.
What is common in the structures of the compounds methonal and ethanol ?
Answer:
They have the same functional group (—OH) known as alcoholic group.
Question 25.
Which functional groups are present in the family of
(i) alcohols
(ii) aldehydes
(iii) carboxylic acids ?
Answer:
(i) —OH
(ii) —CHO
(iii) —COOH.
Question 26.
Identify from the following the hydrocarbons that undergo addition reactions :
C3H4, C2H6, CH4, C2H4. Justify your answer
Answer:
The hydrocarbons are C2H4 (ethene) with formula CnH2n and C3H4 (propyne) with formula CnH2n-2 both are unsaturated.
Question 27.
Which element exhibits the property of catenation to maximum and why ? (CBSE 2016)
Answer:
The element is carbon. This is because of very small size of carbon atom (77 pm) and high strength of C—C bond (355 kj mol-1).
Question 28.
Select the saturated hydrocarbons from the following
C3H6; C5H10; C4H10; C6H14; C2H4 (CBSE 2016)
Answer:
The compounds C4H10 (butane) and C6H14 (hexane) are saturated hydrocarbons. They correspond to the molecular formula CnH2n+2.
Question 29.
Write the molecular formula of the first two members of the homologous series having functional group >C=0. (CBSE 2017)
Answer:
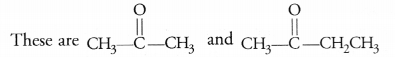
Question 30.
Name the functional group present in the compound CH3CH2CH2COOH.
Answer:
The functional group (—COOH) is known as carboxyl group.
Short Answer Questions
Question 31.
Write the structures of
(i) Ethanoic acid
(ii) Butanone
(iii) Hexanal
(iv) But-2-ene.
Answer:
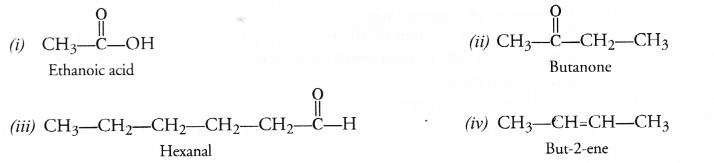
Question 32.
How will you name the following compounds ?
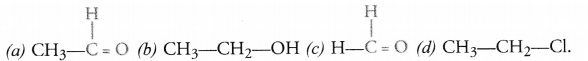
Answer:
(a) Ethanal
(b) Ethanol
(c) Methanal
(d) Chloroethane.
Question 33.
Identify the name of the functional groups in the following compounds.
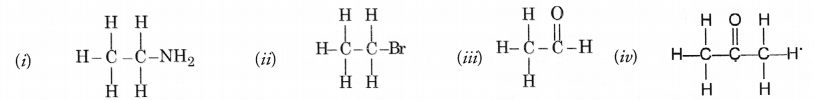
Answer:
(i) —NH2 (amino) (ii) —Br (bromo)
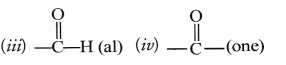
Question 34.
Write the IUPAC names of the following compounds.
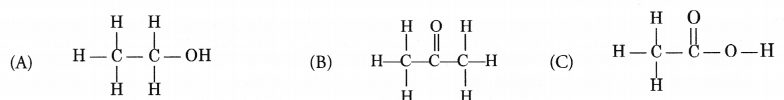
Answer:
(A) Ethanol
(B) Propanone
(C) Ethanoic acid.
Question 35.
Give the electron dot structure and structural formula of first member of alkene and alkyne families.
Answer:

Question 36.
Draw the structural formulae of the possible isomers for the compound with molecular formula C3H6O ?
(CBSE Sample Paper 2017)
Answer:
The given organic compounds represents two structural isomers which are actually functional isomers in nature.
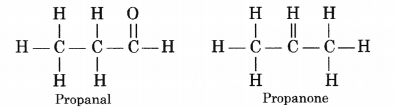
Question 37.
How will you convert ethene into ethanol ? Give the chemical reaction involved.
Answer:
Ethene is converted into ethanol by passing its vapours through water in the presence of sulphuric acid. This reaction is called hydration of ethene.
![]()
Question 38.
What is an homologous series ? Which two of the following organic compounds belong to the same homologous series ?
C2H6, C2H6O, C2H6O2,
Answer:
Homologous series represent different families of organic compounds into which these are divided. Two characteristics of homologous series are listed.
The compounds CH4O and C2H6O belong to the same homologous series known as alkanols.
Question 39.
State two characteristic features of carbon which when put together give rise to a large number of carbon compounds.
Answer:
- The size of carbon atom is very small (Atomic radius = 77 pm)
- The strength C—C bond is quite high (355 kj mol-1)
Therefore, any number of carbon atoms can be linked by covalent bonds, f his self linking property is called catenation.
Question 40.
Why is petrol regarded as a better fuel than kerosene ?
Answer:
In petrol, the combustion of hydrocarbons present is complete and they burn with blue flame. However, in kerosene, the combustion is not complete. It burns with smoky flame accompanied by the release of unburnt carbon atoms. Therefore, petrol is regarded as a better fuel than kerosene.
Question 41.
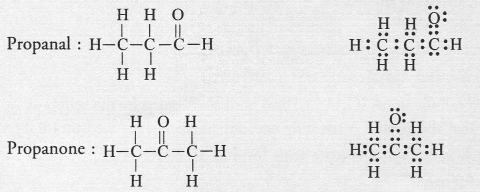
The molecular formula C3H6O can represent an aldehyde as well as ketone. Write their structures and name them. How are they related to each other ?
Answer:
The aldehyde and ketone with formula C3H6O are propanal and propanone. Having the same molecular formula, these are isomers. As the functional groups are different, these are regarded as functional isomers.
For example,
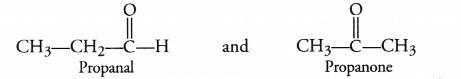
Question 42.
(i) Take about 3mL, of ethanol in a test tube and warm it gently in a water bath.
(ii) Add a 5% solution of alkaline potassium permanganate drop by drop to the solution.
(iii) What happens to the colour of KMnO4 added initially and then in excess ? Give reason. Name the product of this reaction.
Answer:
The purple colour of alkaline potassium permanganate solution, also known as Baeyer’s reagent gets initially discharged. On adding the reagent in excess, the purple colour persists. Actually, Baeyer’s reagent is an oxidising agent. It provides oxygen to oxidise ethanol to ethanoic acid. Once the oxidation is complete, the further addition of Baeyer’s reagent imparts the purple colour to the solution.

Question 43.
Give the names of the following :
(i) An aldelyde derived from methane
(ii) Ketone derived from butane
(iii) The compound obtained by the oxidation of ethanol with chromic anhydride.
Answer:
(i) Methanal (HCHO)
(ii) Butanone (CH3COCH2CH3)
(iii) Ethanal (CH3CHO)
Question 44.
Write chemical equations for the reactions of ethanoic acid with :
(i) sodium
(ii) sodium carbonate
(iii) ethanol in the presence for cone. H2SO4
Answer:
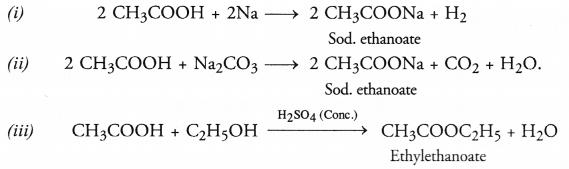
Question 45.
A compound ‘X’ has the molecular formula C3H6O with structural formula CH3CH2CHO. Give its IUPAC name. Can another compound have the same molecular formula ? Give the structure and IUPAC name of that compound also ?
Answer:
The IUPAC name of X is : Propanal. Another compound Y can also have the same molecular formula but different structural formula. It is propanone.

The compounds X and Y are related to each other as functional isomers.
Question 46.
An organic compound ‘X’ has the molecular formula C2H4O2. It has a pleasent smell. It does not turn blue litmus red; nor does it give any effervescence with sodium hydrogen carbonate solution. Predict the com pound. Give its structural formula as well as IUPAC name.
Answer:
Two different structural formulae are possible for the compound ‘X’ with molecular formula C2H4O2. These are known as functional isomers and may be written as :
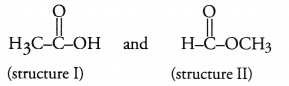
Structure I is that of a carboxylic acid, ethanoic acid. Since the compound ‘X’ does not turn blue litmus red and also does not give effervescence with NaHCO3 solution, it cannot be an acid.
As the compound has a pleasent smell, it seems to be an ester with structure II. Please note that the esters have pleasant smell. The IUPAC name of the compound is methylmethanoate.
Question 47.
Acetic acid was added to a solid ‘X’ kept in a test tube. A colourless and odourless gas was evolved. The gas turned lime water milky when passed through it. Predict the nature of the solid.
Answer:
Since the gas was colourless as well as odourless and turned lime water milky, it is carbon dioxide gas. The solid ‘X’ which has liberated the gas on reacting with acetic acid is either a metal carbonate (e.g. Na2CO3) or some hydrogen carbonate of metal (e.g. NaHCO3)
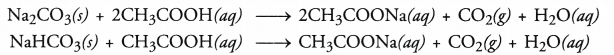
Question 48.
An organic compound A’ is a constituent of antifreeze and has the molecular formula C2H6O. Upon reaction with alkaline KMnO4, the compound A’ is oxidised to another compound ‘B’ with formula C2H6O2. Identify the compounds A and ‘B’. Write the chemical equation for the reaction which leads to the formation of ‘B’.
Answer:
The compound A is ethanol and with alkaline KMnO4 it is oxidised to ethanoic acid ‘B’. The chemical equation for the reaction is :
Question 49.
Name the functional groups present in the following compounds :
(i) CH3—CH2—CH2—OH
(ii) CH3—CH2—CH2—COOH
(iii) CH3—CH2—CHO
(iv) CH3—CO—CH2—CH3
Answer:
(i) —OH (ol)
(ii) —COOH (oic acid)
(iii) —CHO (al)
(iv) —CO— (one)
Question 50.
(a) Draw the structure of the following compounds :
(i) Ethanoic acid
(ii) Butanone.
(b) Why is conversion of ethanol to ethanoic acid considered an oxidation reaction ?
Answer:
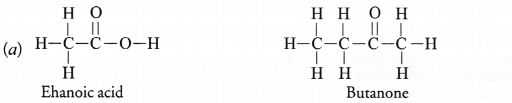
(b) When ethanol (C2H5OH) changes with ehanoic acid (CH3COOH)
- There is a decrease in the number of hydrogen atoms by two.
- There is an increase in the number of oxygen atoms by one.
Therefore, the conversion represents an oxidation reaction.
Question 51.
(a) What are esters ? How are they formed ?
(b) Write two uses of esters ? (CBSE 2017)
Answer:
(a) Esters are the group of organic compounds which contain the function group (—COOR) called ester group. The value of R may change as —CH3, —C2H5, —C3H7, etc. A few examples of esters are

Esters are formed as a result of chemical reaction called esterification.
(b) Uses of esters
- Esters have pleasent smell. These are used as flavouring agents and also in perfumes.
- Esters of glycerol known as triglycerides, are used in the manufacture of soaps.This reaction is called saponification reaction.
Question 52.
Write the names and moleculer formula of two organic compounds having functional group suffixed as ‘-oic acid’.
With the help of a balanced equations, explain what happens when any of them reacts with sodium hydroxide.
Answer:
The organic compounds with functional group-oic acid are known as carboxylic acids or alkanoic acids. These are represented by general formula RCOOH (Where R may be H atom or alkyl group). For example,
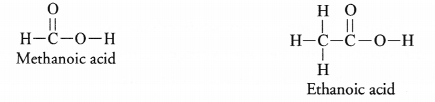
On reacting a carboxylic acid with sodium hydroxide (NaOH), corresponding sodium salt and water are formed. The reaction is known as neutralisation.
![]()
Question 53.
Write the name and molecular formula of an organic compound having its name suffixed with of and having two carbon atoms in the molecule. With the help of a balanced chemical equation indicate what happens when it is heated with excess of cone. H2SO4. (CBSE 2016)
Answer:
The compound is ethanol and its molecular formula is C2H5OH. Upon heating with excess of cone. H2SO4, it loses a molecule of H2O and forms ethene. The reaction is known as dehydration reaction.
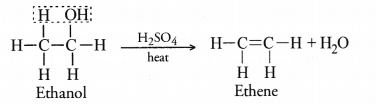
Question 54.
Explain with the help of chemical equations, the following properties of carbon.
(i) Combustion
(ii) Oxidation.
Answer:
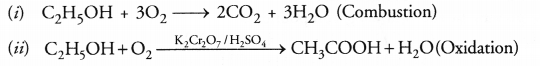
Question 55.
A neutral organic compound A of molecular formula C2H6O on heating with excess of cone. H2SO4 gives compound B of molecular formula C2H4. Compound B on reduction gives compound C of molecular formula C2H6.
(a) Name A, B and C.
(b) Write chemical equation for the conversion of A to B.
(c) What is the role of cone. H2SO4 in the above equation.
Answer:
(a) The compounds A, B and C are ethanol (C3H6O), ethene(C2H4) and ethane(C2H6) respectively.
(b) For the chemical equation,
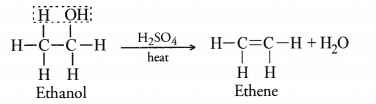
(c) Cone. H2SO4 is used as a dehydrating agent in the reaction.
Long Answer Questions
Question 56.
An organic compound ‘A’ is an essential constituent of wine and beer. Oxidation of ‘A’ yields an organic acid ‘B’ which is present in vinegar. Name the compounds ‘A’ and ‘B’ and write their strucutral formulae. What happens when ‘A’ and ‘B’ react in the presence of an acid catalyst ? Write the chemical equation for the reaction.
(CBSE All India 2010)
Answer:
The available information suggests that the compound ‘A’ is ethanol and the compound ‘B’ formed by the oxidation of ‘A’ is ethanoic acid. Their structural formulae are :

When ‘A’ and ‘B’ react in the presence of an acid like cone. H2SO4, the compound is ethyl ethanoate (ester) with a pleasant smell.
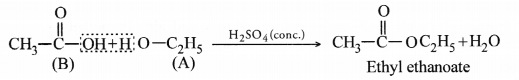
Question 57.
Give a chemical test to distinguish between :
(i) Ethane and ethene
(ii) Ethanol and ethanoic acid
(iii) Soaps and detergents.
Answer:
(i) Ethene decolorises the yellow colour of bromine water while ethane does not.
(ii) Ethanoic acid gives a brisk effervescence with sodium hydrogen carbonate while ethanol does not.
(iii) Soaps form curdy white precipitate or scum with hard water while detergents do not form any precipitate.
Question 58.
(a) What are homologous series of compounds ? List any two characteristics of homologous series.
(b) What would be observed by adding a 5% solution of alkaline potassium permanganate drop by drop to warm ethanol taken in a test tube ? (c) Write the name of the compound formed during the chemical reaction. How would you distinguish experimentally between an alcohol and a carboxylic acid on the basis of a chemical property.
Answer:
(a) Homologous series represent different families of organic compounds into which these are divided. Two characteristics of homologous series are listed.
- All the members in a particular homologous series of family have the same characteristic functional group. For example, in organic acids, the functional group is carboxyl group (—COOH).
- Any two consecutive members in a particular family have the same common difference of CH2 in their molecular formulae. For example, the first three members of the family of alkanes are : CH4 (methane), C2H6 (ethane) and propane (C3H8).
(b) On adding a 5% solution of alkaline potassium permanganate to ethanol, it will be oxidised to ethanoic acid.
The pink colour of the solution will get discharged upon warming.
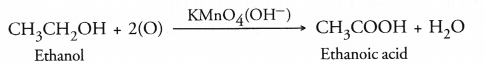
(c) A carboxylic acid gives a brisk effervescence when an aqueous solution of sodium hydrogen carbonate (NaHCO3) is added to it. This is due to the evolution of CO2 gas. However, alcohol will not give any reaction.
Question 59.
(a) How are carboxylic acids different from mineral acids from ionisation point of view ?
(b) Describe an activity to show how ethanoic acid reacts with sodium carbonate. Name the gas evolved. How can it be tested ?
(c) State the principle on which the cleansing action of soap is based.
Answer:
(a) Carboxylic acids (organic acids) are less ionised in solution as compared to mineral acids (HCl, HNO3, H2SO4 etc.) Due to this reason, these are weaker acids than the mineral acids.
(b) Take a small volume of ethanoic acid in a tube. Add a few drops of sodium carbonate (Na2CO3) solution prepared in water to the tube. A colourless gas with brisk effervescence will evolve. When the gas is passed through lime water, it will become milky.
(c) The cleansing action of soap is based on its tendency to act as a bridge between water and oil drops containing dirt particles. As a result, oil and water get mixed. They form a stable emulsion also called micelle. This helps in removing oil drops containing dirt particles from clothes. The clothes become clean.
Question 60.
(a) Distinguish between esterification and saponification reactions of organic compounds.
(b) With the help of a labelled diagram, describe an activity to show the formation of an ester.
Answer:
(a) In the esterification reaction an acid reacts with alcohol in the presence of cone. H2SO4 to form an ester with a pleasant or fruity smell. For example,
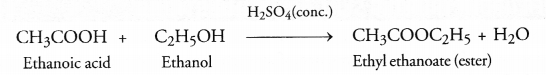
Saponification is quite different from esterification because in this case an ester reacts with an alkali (NaOH or KOH) to form salt of acid and alcohol. For example,

(b) For the activity,
Esters can be easily formed in the laboratory also. Take equal volumes of ethyl alcohol and glacial acetic acid (say 2 mL) alongwith a few drops of concentrated sulphuric acid in a test tube. In the mean time, warm water in a beaker as shown in the figure. Keep the test tube in warm water for some time. You will experience a pleasant smell. This shows that ethyl acetate (ester) has been formed in the reaction.
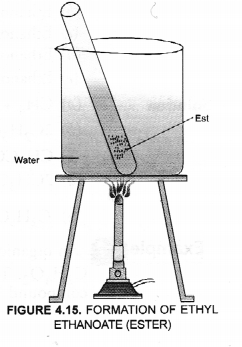
Esters as pointed, are pleasant smelling compounds. These are therefore, commonly used as flavouring agents and also in perfumes. When an ester is reacted with water in the presence of a dilute acid like dilute HCl, acid and alcohol are formed as the product. The reaction is called ester hydrolysis.
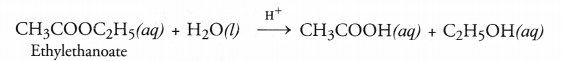
Ester hydrolysis is the reverse of esterification reaction.
When an ester is reacted with an aqueous solution of base like NaOH or KOH, the product is an alcohol and salt of the acid. For example,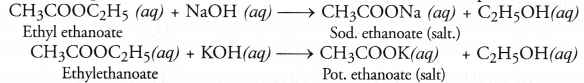
The reaction is known as saponification reaction because it is the basis for the formation of soap.
Question 61.
An ester has the molecular formula C4H8O2. Write its structural formula. What happens when this ester is heated in the presence of sodium hydroxide solution ? Write the balanced chemical equation for the reaction and name the products. What is a saponification reaction ?
Answer:
For the molecular formula C4H8O2, two isomeric esters are possible which differ in structural formulae.
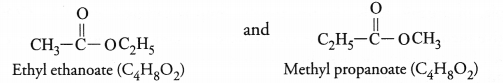
Both will react with sodium hydroxide upon heating to form the sodium salt of the acid and alcohol.
Both the reactions are the examples of saponification reactions. In these, an ester reacts with an alkali upon heating to form corresponding salt and alcohol.
Question 62.
Two carbon compounds ‘A’ and ‘B’ have the molecular formula C3H8 and C3H6 respectively. Which one of the two is most likely to show addition reactions ? Justify your answer. Explain with the help of a chemical equation, how an addition reaction is useful in vegetable ghee industry.
Answer:
The compound ‘A’ with formula C3H8(propane) is a saturated hydrocarbon and corresponds to general formula CnH2n+2. The compound ‘B’ with formula C3H6 (propene) is an unsaturated hydrocarbon and corresponds to general formula CnH2n. It has a double bond (C=C) and is therefore, unsaturated.
The compound ‘B’ will take part in the addition reactions. As a result, double bond will change to single bond. For example,
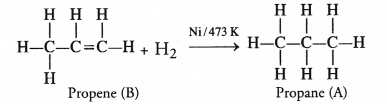
The addition reaction is quite useful in the hydrogenation of oils i.e., to convert edible oils like ground nut oil and cotton seed oil which are unsaturated in nature into solid fats which are of saturated nature.
NCERT Exemplar Solutions for Class 10 Science Chapter 4 Carbon and Its Compounds
NCERT Exemplar Solutions for Class 10 Science Chapter 4 Multiple Choice Questions
Question 1.
Carbon exists in the atmosphere in the form of
(a) carbon monoxide only
(b) carbon monoxide and carbon dioxide in traces
(c) carbon dioxide only
(d) coal gas.
Answer:
(b). Both CO and CO2 gases are present in traces in the atmosphere.
Question 2.
Which of the following statements are usually correct for carbon compounds ? These
(i) are good conductors of heat and electricity
(ii) are poor conductors of electricity
(iii) have strong forces of attraction between their molecules
(iv) do not have strong forces of attraction between their molecules
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer:
(d). Carbon compounds are of covalent nature. That is why these are poor conductors of electricity and do not have strong forces of attraction.
Question 3.
A molecule of ammonia (NH3) has
(a) only single bonds
(b) only double bonds
(c) only triple bonds
(d) two double bonds and two single bonds.
Answer:
(a). All bonds are single covalent in nature.
Question 4.
Buckminster fullerene is an allotropie form of
(a) nitrogen
(b) sulphur
(c) carbon
(d) tin
Answer:
(c).
Question 5.
Which of the following are correct chain isomers of butane ?
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:
(c). Isomer (i) has a straight chain while (ii) is a branched chain isomer.
Question 6.
![]()
In the above reaction, alkaline KMnO4 acts as
(a) reducing agent
(b) oxidising agent
(c) catalyst
(d) dehydrating agent.
Answer:
(b). Alkaline KMnO4 is also known as Baeyer’s reagent. It acts as an oxidising agent.
Question 7.
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
(a) Addition reaction
(b) Substitution reaction
(c) Displacement reaction
(d) Oxidation reaction
Answer:
(a). Oils are unsaturated in nature. By the addition of hydrogen, they become saturated.
Question 8.
In which of the following compounds, —OH is the functional group ?
(a) Butanone
(b) Butanol
(c) Butanoic acid
(d) Butanal
Answer:
(b). —OH is the functional group in butanol which is an alcohol.
Question 9.
The soap molecule has a
(a) hydrophilic head and a hydrophobic tail
(b) hydrophobic head and a hydrophilic tail
(c) hydrophobic head and a hydrophobic tail
(d) hydrophilic head and a hydrophilic tail
Answer:
(a). ‘Head’ is a polar group and is attacked towards H2O molecules of water. ‘Tail’ is a long chain of hydrocarbons and is water repellent.
Question 10.
Which of the following is the correct representation of electron dot structure of nitrogen ?
![]()
Answer:
(d). Both the N atoms have a complete octet after electron sharing.
Question 11.
Structural formula of ethyne is

Answer:
(a).
Question 12.
Identify the unsaturated compounds from the following
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (ii) and (iii)
Answer:
(d). Both propene and propyne are unsaturated hydrocarbons.
Question 13.
Chlorine reacts with saturated hydrocarbons at room temperature in the
(a) absence of sunlight
(b) presence of sunlight
(c) presence of water
(d) presence of hydrochloric acid
Answer:
(b). These reactions are called photochemical reactions.
Question 14.
In the soap micelles
(a) the ionic end of soap is on the surface of the cluster while the carbon chain is in the interior of the cluster.
(b) ionic end of soap is in the interior of the cluster and the carbon chain is out of the cluster,
(c) both ionic end and carbon chain are in the interior of the cluster
(d) both ionic end and carbon chain are on the exterior of the cluster
Answer:
(a).
Question 15.
Pentane has the molecular formula C5H12. h has
(a) 8 covalent bonds
(b) 10 covalent bonds
(c) 16 covalent bonds
(d) 14 covalent bonds
Answer:
(c).
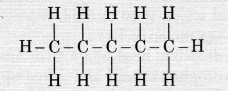
Question 16.
Structural formula of benzene is
Answer:
(c). The carbon atom skeleton in benzene has alternate single and double bonds
Question 17.
Ethanol reacts with sodium and forms two products. These are :
(a) sodium ethanoate and hydrogen
(b) sodium ethanoate and oxygen
(c) sodium ethoxide and hydrogen
(d) sodium ethoxide and oxygen
Answer:
(c).
![]()
Question 18.
The correct structural formula of butanoic acid is
Answer:
(d).
Question 19.
Vinegar is a solution of
(a) 30% — 40% acetic acid in alcohol
(b) 5% — 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 15% – 20% acetic acid in water
Answer:
(c).
Question 20.
Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised
(ii) carboxylic acids are completely ionised
(iii) mineral acids are partially ionised
(iv) carboxylic acids are partially ionised
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:
(a). Mineral acids like HCl are completely ionised in solvent like water whereas carboxylic acids such as CH3COOH are only partially ionised.
Question 21.
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
(a) helium
(b) neon
(c) argon
(d) krypton
Answer:
(b). The compound formed is methane (CH4). In this, carbon atom has a complete octet and configuration of neon which is a noble gas element.
Question 22.
The correct electron dot-structure of a water molecule is
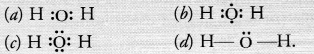
Answer:
(c).
Question 23.
Which of the following is not a straight chain hydrocarbon ?
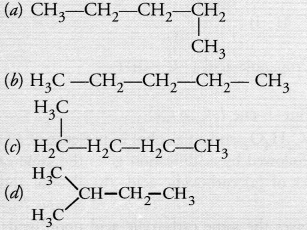
Answer:
(d). Please note that the continuous chains of carbon atoms whether straight or bent are not branched in nature.
Question 24.
Which among the following are unsaturated hydrocarbons ?
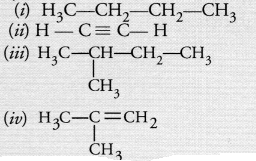
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv).
Answer:
(c). Both (ii) and (iv) are unsaturated in nature.
Question 25.
The IUPAC name of the compound
CH3—CH2—CHO is
(a) Propanal
(b) Propanone
(c) Ethanol
(d) Ethanal
Answer:
(a).
Question 26.
The heteroatoms present in CH3—O—CH2—CH2 (Br) are
(i) oxygen
(ii) carbon
(iii) hydrogen
(iv) bromine
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer:
(d). Both oxygen (O) and bromine (Br) are heteroatoms. Please remember that apart from C and H atoms, all other atoms present in an organic compound are hetero atoms.
Question 27.
Which of the following does not belong to the same homologous series ?
(a) CH4
(b) C2H6
(c) C3H8
(d) C3H6.
Answer:
(d). It is an alkene while the rest are all alkane molecules in nature.
Question 28.
The first member of alkene family is
(a) ethyne
(b) ethene
(c) propyne
(d) ethane
Answer:
(b).
Question 29.
Which of the following represents saponification
Answer:
(d). This is an example of saponification reaction. All other options are not correct.
NCERT Exemplar Solutions for Class 10 Science Chapter 4 Short Answer Questions
Question 30.
Draw the electron dot structure of ethyne and also draw its structural formula
Answer:
![]()
Question 31.
Write the names of the following compounds :

Answer:
(a) Pentanoic acid
(b) But-l-yne
(c) Heptanal
(d) Pentan-l-ol
Question 32.
Identify and name the functional groups present in the following compounds.


Answer:

Question 33.
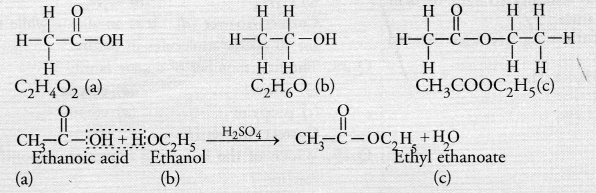
A compound X is formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of (a) carboxylic acid,
(b) alcohol and
(c) the compound X. Also write the reaction. (CBSE Sample Paper 2017)
Answer:
The available information suggests that the alcohol which gives the same carboxylic acid upon oxidation has two carbon atoms. It is, therefore ethanol (C2H5OH). The structures of the different compounds are :
Question 34.
Why are detergents better cleansing agents than soaps ? Explain.
Answer:
A substance capable of removing grease and dirt from any fabric or body is called detergent. The detergents are of two types Le. soapy and non-soapy detergents. The soapy detergents are soaps whereas the non-soapy detergents are synthetic detergents or simply detergents. Although both are cleansing agents, they differ in chemical composition. In the present chapter, we shall briefly discuss the composition and cleansing action of soaps and synthetic detergents.
Soaps are the sodium and potassium salts of long chain fatty acids with general formula RCOONa or RCOOK. The acids present have the formula RCOOH where R may have following values.
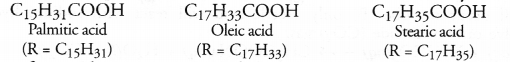
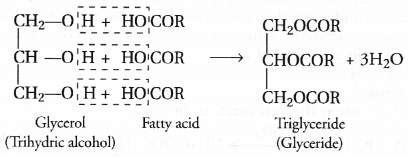
These fatty acids exist as triesters of glycerol which is a trihydric alcohol. The triesters are also called triglycerides or simply glycerides and are the constituents of edible oils and fats. These are of animal and vegetable origin e.g. castor oil, linseed oil or soyabean oil. Chemically the triglycerides are formed as a result of esterification reaction.
Question 35.
Identify the functional groups present in the following compounds
![]()
Answer:
(a) >C = O
(b) —COOH
(c) —CHO
(d) —OH.
Question 36.
How is ethene prepared from ethanol ? Give the reaction involved in it.
Answer:
From ethanol: This method involves the slow oxidation of a dilute solution of ethanol (10-15 per cent) by oxygen present in air in the presence of an enzyme acetobactor.

The acid obtained is in the form of dilute solution called vinegar.
We have also studied under ethanol that it gets oxidised to ethanoic acid in the presence of dilute solution of alkaline KMnO4 or acidified K2Cr2O7.
Question 37.
Intake of small quantity of methanol can be lethal. Comment.
Answer:
From methanol: These days ethanoic acid is manufactured by the reaction between methanol and carbon monoxide in the presence of iodine-rhodium (I2 — Rh) catalyst mixture.

Question 38.
A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation for the reaction involved.
Answer:
The gas evolved is hydrogen. The balanced chemical equation for the reactibn is :
![]()
Question 39.
Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid. What is the role of sulphuric acid in this reaction ? Write the balanced chemical equation of this reaction.
Answer:
Concentrated sulphuric acid acts as a dehydrating agent in the reaction. Ethene is formed as the
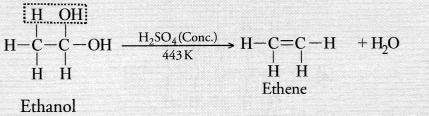
Question 40.
Carbon, Group (14) element in the Periodic Table, is known to form compounds with many elements. Write an example of a compound formed with
(a) chlorine (Group 17 of Periodic Table)
(b) oxygen (Group 16 of Periodic Table)
Answer:
(a) The compound is carbon tetrachloride (CCl4)
(b) The compound is carbon dioxide (CO2)
Question 41.
In electron dot structure, the valence shell electrons are represented by crosses or dots.
(a) The atomic number of chlorine is 17. Write its electronic configuration
(b) Draw the electron dot structure of chlorine molecule.
Answer:
(a) 2, 8, 7
![]()
Question 42.
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
Answer:
The tendency to show catenation is very small in case of Si as compared to C although both the elements belong to the same group (14). This is because of greater atomic size of Si atom (118 pm) than that of carbon (77 pm). As a result, the strength of Si—Si bond is less as compared to that of C—C bond. This means that lesser number of Si atoms can be linked to each other by covalent bonds as compared to carbon atoms.
Question 43.
Unsaturated hydrocarbons contain multiple bonds between the two C-atoms and show addition reactions. Give the test to distinguish ethane from ethene.
Answer:
Distinction between ethane and ethene can be done with the help of bromine water test. Whereas ethene dicolourises the yellow colour of bromine water, ethane does not.
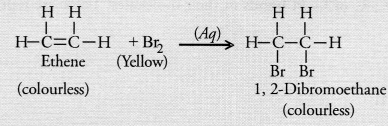
Question 44.
Match the reactions given in Column (A) with the names given in column (B).
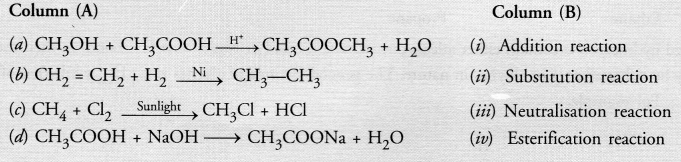
Answer:
(a)—(iv) ;
(b)—(i) ;
(c)—(ii) ;
(d)—(iii)
Question 45.
Write the structural formulae of all the isomers of hexane.
Answer:
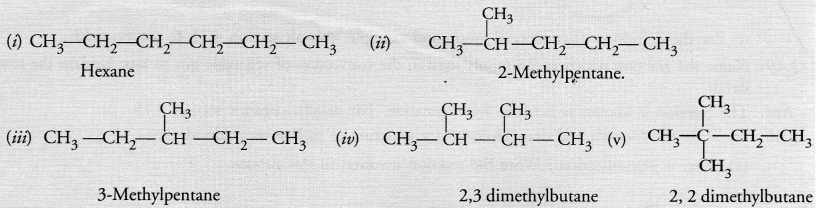
Question 46.
What is the role of metal or reagents written on arrows in the given chemical reactions?
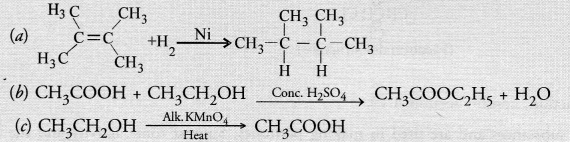
Answer:
(a) Nickel (Ni) acts as a hydrogenation catalyst for the reaction
(b) Cone. H2SO4 removes a molecule of H2O from the reaction mixture and acts as a dehydrating agent.
(c) Alkaline KMnO4 acts as an oxidising agent and oxidises ethanol to ethanoic acid.
NCERT Exemplar Solutions for Class 10 Science Chapter 4Long Answer Questions
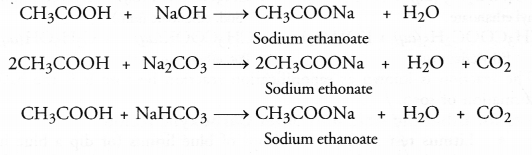
Question 47.
A salt X is formed and a gas is evolved when ethanoic acid reacts with sodium hydrogen carbonate. Name the salt X and the gas evolved. Describe an activity and draw the diagram of the apparatus to prove that the evolved gas is the one which you have named. Also, write chemical equation of the reaction involved.
Answer:
The salt X formed in the reaction is sodium ethanoate. The gas evolved is carbon dioxide gas. For the activity and chemical reaction,
Question 48.
(a) What are hydrocarbons ? Give examples.
(b) Give the structural differences between saturated and unsaturated hydrocarbons with two examples of each.
(c) What is a functional group ? Give examples of four different functional groups.
Answer:
(a) Hydrocarbons are the organic compounds containing only carbon and hydrogen atoms as their constituents. These may be alkanes, alkenes and alkynes.
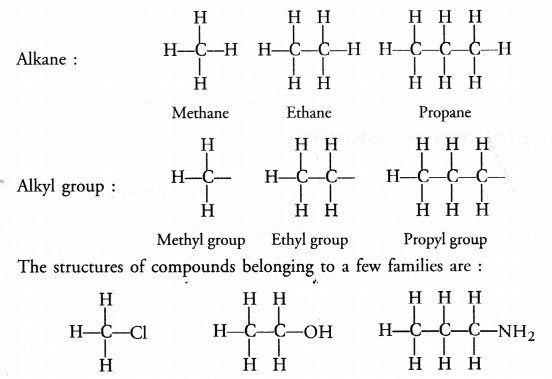
(b) Saturated hydrocarbons or alkanes contain either C—C or C—H bonds in their molecules. These are represented by the general formula CnH2n+2 For example,
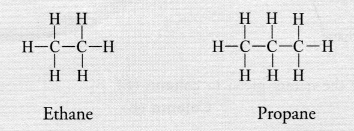
Unsaturated hydrocarbons contain either atleast one >C = C< bond or triple —C ≡ C— bond in their molecules. These may be either alkenes or alkynes in nature. The general formula of alkenes is CnH2n while that of alkynes is CnH2n-2 for example,
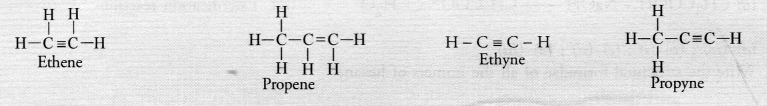
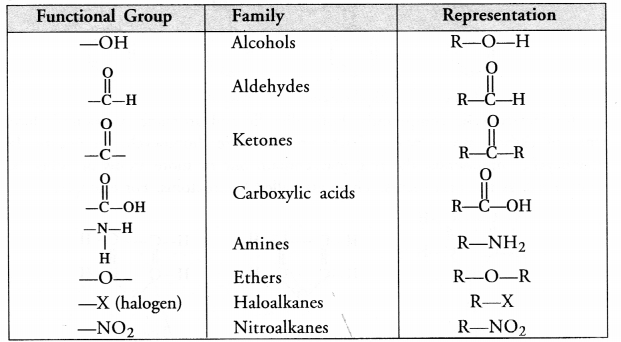
(c) For the definition of functional group and example,
Question 49.
Name the reaction which is commonly used in the conversion of vegetable oils to fats. Explain the reaction in detail.
Answer:
The reaction is known as catalytic hydrogenation. For details, constult section 4.15.
Question 50.
(a) Write the formula and draw electron dot structure of carbon tetrachloride.
(b) What is saponification ? Write the reaction involved in this process.
Answer:

(b) For saponification reaction,
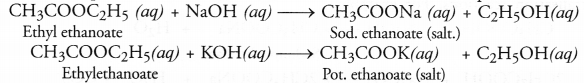
The reaction is known as saponification reaction because it is the basis for the formation of soap.
Question 51.
Esters are sweet-smelling substances and are used in making perfumes. Suggest some activity and the reaction involved for the preparation of an ester with well labelled diagram.
Answer:
Esters as pointed, are pleasant smelling compounds. These are therefore, commonly used as flavouring agents and also in perfumes. When an ester is reacted with water in the presence of a dilute acid like dilute HCl, acid and alcohol are formed as the product. The reaction is called ester hydrolysis.
Ester hydrolysis is the reverse of esterification reaction.
When an ester is reacted with an aqueous solution of base like NaOH or KOH, the product is an alcohol and salt of the acid. For example,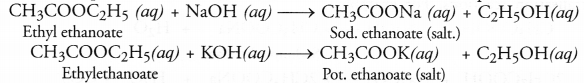
The reaction is known as saponification reaction because it is the basis for the formation of soap.
Question 52.
A compound C (molecular formula, C2H4O2) reacts with Na-metal to form a compound R and evolves a gas which burns with a pop sound. Compound C on treatment with an alcohol A in presence of an acid forms a sweet smelling compound S (molecular formula, C3H6O2). On addition of NaOH to C, it also gives R and water. S on treatment with NaOH solution gives back R and A.
Identify C, R, A, S and write down the reactions involved.
Answer:
From the available information, it is evident that
- Compound C with molecular formula C2H4O2 is ethanoic acid (CH3COOH)
- The compound R is sodium ethanoate and has formula CH3COONa
- Since the compound S has only three carbon atoms (C3H6O2) and has been formed by the action of an alcohol on compound C (C2H4O2), this means that the alcohol A has only one carbon atom. It is methanol (CH3OH).
- The compound S with a sweet smell is methyl ethanoate with formula CH3COOCH3.
The chemical reactions involved are as follows :
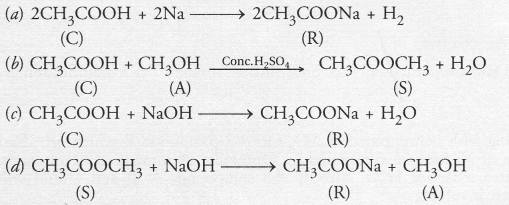
Question 53.
Look at given figure and answer the following questions :
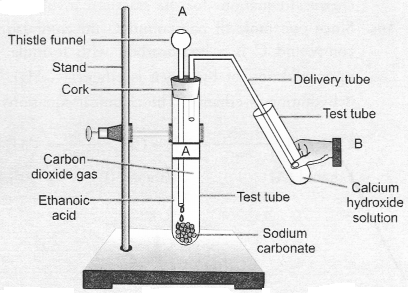
(a) What change would you observe in the calcium hydroxide solution taken in tube B ?
(b) Write the reaction involved in test tubes A and B respectively.
(c) If ethanol is used instead of ethanoic acid, would you expect the same change ?
(d) How can a solution of lime water be prepared in the laboratory ?
Answer:
(a) It would become milky.
In test tube B, calcium hydroxide reacts with carbon dioxide to form calcium carbonate which is milky in colour.
![]()
(c) No, it would be different. No chemical reaction is possible between ethanol and sodium carbonate.
(d) Lime w’ater is prepared by keeping suspension of calcium hydroxide overnight in a beaker. The solution is decanted and is transferred to another beaker. It contains traces of calcium hydroxide and is called lime water.
Question 54.
How would you bring about the following conversions ? Name the process and write the reaction involved.
(a) ethanol to ethene.
(b) Methanol to Ethanoic acid. Write the reactions.
Answer:
(a) From ethanol: This method involves the slow oxidation of a dilute solution of ethanol (10-15 per cent) by oxygen present in air in the presence of an enzyme acetobactor.

The acid obtained is in the form of dilute solution called vinegar.
We have also studied under ethanol that it gets oxidised to ethanoic acid in the presence of dilute solution of alkaline KMnO4 or acidified K2Cr2O7.
(b) From methanol: These days ethanoic acid is manufactured by the reaction between methanol and carbon monoxide in the presence of iodine-rhodium (I2 — Rh) catalyst mixture.

Question 55.
Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures.
Answer:
Two isomers are possible for the molecular formula C3H6O. These are known as functional isomers.
Question 56.
Explain the given reactions with the examples :
(a) Hydrogenation reaction
(b) Oxidation reaction
(c) Substitution reaction
(d) Saponification reaction
(e) Combustion reaction
Answer:
Hydrogenation of oils : The reaction is extremely useful in the hydrogenation of vegetable oils also called edible oils e.g. ground nut oil, cotton seed oil etc. These are also called cooking oils and are unsaturated in the sense that their molecules contain atleast one C=C bond in their structures. Upon passing hydrogen gas through oil in the presence of nickel catalyst, the double bond changes to single bond. As a result, the unsaturated oil changes to solid fat which is of saturated nature. Vegetable ghees such as Dalda, are of saturated nature and are formed by catalytic hydrogenation reaction.
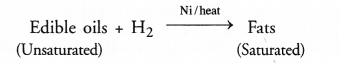
Oxidation reaction :
- Loss of hydrogen is known as oxidation.
- Gain of oxygen is known as oxidation.
Therefore, it is an oxidation reaction.
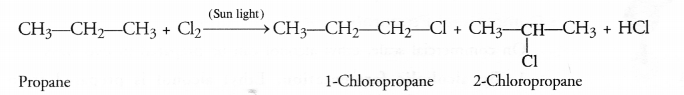
Substitution Reactions : Substitution reactions are also called replacement reactions and you are quite familiar with these. In organic compounds, particularly the saturated hydrocarbons (or alkanes), these reactions are very common. One or more hydrogen atoms in the molecule of alkane such methane get substituted by chlorine atoms when the reaction is carried with chlorine in the presence of ultraviolet sun light.
Combustion reaction :
We have seen that all combustion reactions are basically oxidation reactions carried in the presence of air or oxygen. It is not necessary that the reactants may burn during combustion.
- Blue flame signifies complete combustion of the fuel.
- Yellow sooty flame signifies incomplete combustion of the fuel.
Question 57.
An organic compound A on heating with concentrated H2SO4 forms a compound B which on addition of one mole of hydrogen in presence of Ni forms a compound C. One mole of compound C on combustion forms two moles of CO2 and three moles of H2O. Identify the compounds A, B and C and write the chemical equations for the reactions involved.
Answer:
Since one mole of compound C on combustion forms two moles of CO2 and three moles of H2O the compound C is a hydrocarbon with formula C2H6. It is ethane. The compound B which forms C2H6 upon addition of hydrogen is ethene (C2H4). The organic compound A which forms ethene upon acidic dehydration is ethanol. The chemical equations for the reactions involved are :